A Brief Assessment of Drosophila suzukii (Diptera: Drosophilidae) Abundance in Forest and Non-Forested Habitats Across an Altitude Gradient on Mauna Loa, Hawai‘i1
Drosophila suzukii (Diptera: Drosophilidae) is a significant pest of wild and cultivated soft-skinned fruits. D. suzukii was first detected outside of its native range in 1983 on Mauna Ka‘ala in Wai‘anae, Hawai‘i, and has since spread throughout North America, South America, and Europe. While D. suzukii is not considered a crop pest in Hawai‘i, little data is available on the distribution of the species on a landscape scale on the archipelago. In this study, we document the distribution and abundance of D. suzukii and characterize its host use of ‘ōhelo (Vaccinium reticulatum) across an altitude gradient on the eastern slope of Mauna Loa, Hawai‘i. In total we collected 2,503 D. suzukii across 14 field sites over a four-month period in 2016. Endemic ‘ōhelo is a host for D. suzukii as we detected adult emergence across field sites with up to 1.88 flies per 1 mL of berries. Our preliminary population data shows that D. suzukii abundance is greater at higher altitudes and in forested habitats on Mauna Loa. Given the population abundance of D. suzukii and their ability to use at least one of the three endemic Vaccinium in Hawai‘i as a host, further research on host–use interactions with native and non-native insects is warranted.
Spotted-Wing Drosophila, altitude gradient, ‘ōhelo, Vaccinium reticulatum, invasive species
Invasive species are a significant burden on the world economy, especially for food and timber security (Pimentel et al. 2005). The Spotted Wing Drosophila, Drosophila suzukii (Matsumura 1931) (Diptera: Drosophilidae) is a globally distributed invasive species and is an insect pest of wild and cultivated soft-skinned fruits (Lee et al. 2015a). Drosophila suzukii has a serrated ovipositor that enables females to lay their eggs in ripening fruit, thus inflicting significant damage to the fruit host (Lee et al. 2015b). It is within the fruit that D. suzukii undergoes larval and pupal development, ultimately emerging as an imago (Poyet et al. 2014). Damage to the fruits of important crops like blueberries, cherries, and peaches by D. suzukii are particularly challenging for growers and farmers as they reduce the fruit’s marketability (Walsh et al. 2011). Originating in Asia, D. suzukii was first reported outside of its native range on Mauna Ka‘ala in Wai‘anae, Hawai#i, USA (Kaneshiro 1983). Since 2008, D. suzukii has spread throughout North [End Page 513] America, South America, Europe, and Réunion Island (Fraimout et al. 2017). While extensive research on the distribution and management of D. suzukii has been pursued in continental landscapes, little research has been conducted on the invasion dynamics of D. suzukii across the Hawaiian archipelago.
Adventive species often occupy niche spaces that are climatically distinct from their native range following an introduction to a novel environment (Broennimann et al. 2007). One hypothesis for this expansion is that species may invade environments that are more climatically favorable for reproductive activities (Colautti and Barrett 2013). D. suzukii has found success in invading temperate regions of North America and Europe as it is innately predisposed to invasion success in temperate climates (Ometto et al. 2013, Shearer et al. 2016, Stockton et al. 2019a,b). However, invasive populations of D. suzukii have also flourished in warmer climates such as Hawai‘i, Réunion Island, California, Georgia, and Florida (Kaneshiro 1983, Leblanc et al. 2013, Iglesias et al. 2014, Fraimout et al. 2017, Jaffe et al. 2018, Stockton et al. 2018, 2019a,b, 2020). D. suzukii reproduces in warm seasons in temperate climates when fruit hosts are available and can survive the winter as a cold-tolerant, sexually immature stage (Ometto et al. 2013, Shearer et al. 2016, Stockton et al. 2019a,b). Furthermore, photo-periods below 14 h of day length are linked to reduced egg maturation among female D. suzukii (Wallingford et al. 2016). Given the species’ preference for warm climate and longer daylight for reproductive activities, tropical environments open year-round reproduction opportunities for D. suzukii in newly invaded environments like Hawai#i (Iglesias et al. 2014, Stockton et al. 2018, 2019a,b, 2020).
Hawai‘i hosts an ideal climate for year-round population growth for invasive species. The archipelago’s favorable climate and biological colonization history has resulted in the establishment of more than 9,000 non-native species from all over the globe (http://www.hear.org/) (Asner et al. 2008). Many of these non-native species cause significant harm to native and endemic species of Hawai‘i (Simberloff and Von Holle 1999). Furthermore, non-native insects also cause significant damage to agricultural and ornamental plants in Hawai‘i. There are several non-native flies in the Tephritidae that are serious pests to agriculture in Hawai‘i including the Melon fly [Bactrocera curcurbitae (Coquillet, 1849)], Oriental fruit fly [Bactrocera dorsalis (Hendel, 1912)], and the Mediterranean fruit fly [Ceratitis capitata (Wiedemann, 1824)] (Vargas et al. 2010). Given the significant impact of non-native species on Hawaiian biodiversity and agriculture, characterizing the distribution and host–use of invasive D. suzukii is important for the conservation and management of native insects and plants.
While extensive documentation on the damage of D. suzukii upon soft-skinned fruit crops is available across the globe (Walsh et al. 2011), little is known about the usage frequency of non-crop host plants in Hawai‘i (Magnacca et al. 2008). However, there is an abundance of evidence that D. suzukii will use diverse non-crop host plants and non-fruit diets such as bird manure and mushrooms to rear offspring when preferred resources are scarce (Bal et al. 2017, Tonina et al. 2018, Stockton et al. 2019a,b). With high levels of plant and insect endemicity present in Hawai#i (Wagner et al. 1999, Nishida 2002), including nearly 1,000 described native/endemic Drosophila species (Magnacca et al. 2008), D. suzukii has the potential to compete with native and non-native insects for berry hosts (Leblanc et al. 2009, 2013, Shaw et al. 2018). Therefore, D. suzukii will likely continue to expand throughout habitats that are composed of both native and non-native plant and insect communities (Koch et al. 2020).
D. suzukii is an abundant invasive insect across the Hawaiian archipelago (Magnacca et al. 2008, Leblanc et al. 2009, 2013). Characterizing how populations respond to habitat diversity will provide insight into where D. suzukii may be interacting with native species. In this study, we examine the distribution of D. suzukii across an altitude gradient on Mauna Loa in Hawai‘i over the course of four months. Previous research has detected D. suzukii in a diversity of habitat [End Page 514] types throughout Hawai‘i (Leblanc et al. 2009, 2013). However, little is known about the relationship between population abundance and habitat type, especially in landscapes dominated by native biodiversity. We test the specific hypothesis that D. suzukii abundance is predicted by habitat, namely the intersection between altitude and habitat type. We also determine emergence percentage of a known host of the species in Hawai‘i, ‘ōhelo (Vaccinium reticulatum), at different sites across an altitude gradient. The results of our preliminary field study will provide a baseline into how D. suzukii has invaded novel habitats for more than 30 years in Hawai‘i.
Drosophila suzukii sampling sites along an altitudinal gradient in Hawai‘i Volcanoes National Park. Inset map shows the location of the study area in the Hawaiian archipelago.
materials and methods
Field Study
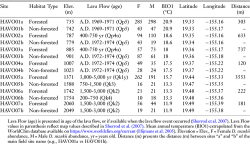
To characterize habitat-use by invasive populations of D. suzukii in Hawai‘i, we surveyed seven paired field sites along an altitude gradient (735–2050 m) on Mauna Loa in Hawai‘i Volcanoes National Park (HAVO) (Figure 1) (Table 1). However, HAVO5 sites a and b are found at 1371 m and 1588 m, respectively (Figure 1), as no field sites following our study design were naturally replicable in the area. Each paired field site represented a forested and non-forested habitat located at a similar altitude and are in close proximity to each other (median = 303 m, maximum = 3553 m, minimum = 120 m) (Figure 1) (Table 1). The forested sites are defined as a site with ≥80% plant cover. To assess plant cover, we used the point-line intercept method to survey the number of plant species detected at one meter (Mueller-Dombois and Ellenberg 1974). At each field site, three Haviland traps were baited with a mixture of ~7 g of yeast, ~14 g of sugar, and 250 mL of water (Lee et al. 2012). The Haviland traps were placed at a height of [End Page 515]
Description of Field Sites Surveyed for Drosophila suzukii on Mauna Loa, Hawai‘i
1.5–2 m and deployed for 4–36 days. All collected flies were placed into 95% EtoH. Samples were returned to the lab and identified and sexed.
To characterize host use by D. suzukii, we hand-picked ‘ōhelo berries at six of the seven non-forested field sites (Figure 1) (sites HAVO1, HAVO2, HAVO3, HAVO4, HAVO6, HAVO7). Berries were collected on multiple occasions in March and April 2016. ‘Ohelo was not present at the non-forested HAVO5. We attempted to collect berries on 5–10 plants at each field site during each visit. We did not detect ‘ōhelo in the forested field sites, and thus no berries could be collected. At least 100 mL of berries from each field site per sampling event was placed in 16 oz containers and covered with a fine mesh secured with a rubber band. Berries were checked daily for insect emergence for up to 30 days. To calculate emergence percentage, we divided the total number of D. suzukii emerged by the total mL of berries collected at a field site and multiplied the value by 100.
D. suzukii Abundance Model
We developed a global model using a generalized linear modeling (GLM) approach to test for the effect of altitude (m), habitat type (forested vs non-forested), Julian day, and trap deployment time on D. suzukii abundance. Models were developed and evaluated using the car and MuMIn libraries in the R statistical programming language (R Core Development Team 2018). Julian day was calculated with the date library in R. D. suzukii abundance represents the count of individuals collected during a trap deployment event. The length of trap deployment was variable across sites and time due. However, rather than standardize trap rate (number of D. suzukii collected/number of deployment days), we elected to include trap deployment time as an independent variable in our analysis. Trap deployment time is the number of days a Haviland trap was left in the field. In the global model, we used the Poisson link function as the data followed a Poisson distribution.
To account for overdispersion in the model, we used the quasi-Poisson link function [End Page 516] in our final global model (Ver Hoef and Boveng 2007). Overdispersion was estimated by calculating ĉ (residual deviance/degrees of freedom), where ĉ values close to 1 imply that the model is not overdispersed. To determine if any of the observations significantly influenced the model parameter estimates we used the function influenced.measures that computes some of the regression diagnostics of GLMs discussed by Belsley et al. (1980) and Cook and Weisberg (1982). Observations that significantly influenced β parameter estimates were removed from the model, with the model subsequently updated with the compareCoefs function in the car library. β ± SE of the updated and non-updated model were compared to determine if removal of an observation influenced parameter estimates and reduced ĉ.
We used the function dredge to examine all possible combinations of the models based on the β in the global model. As the final global model was constructed using the quasi-Poisson link function, we calculated the quasi-Poisson Akaike’s Information Criterion (QAIC), corrected for small sample sizes (QAICc). The QAICc can determine which combination of model parameters best explains D. suzukii abundance in the global model. The QAICc guided approach to model selection is useful as it penalizes the model when new β are added, thus enforcing parsimony (Aho et al. 2014). Models within two QAICc are more or less equivalent and considered top competing models. To further determine the best model of our data, we also calculated QAICc weights (wi), which is the probability of each model given the data and set of models. Thus, larger values of wi, suggest higher support of the model by the data, whereas smaller values of wi suggest lower support of the model by the data. Data and R code used for this analysis are available here: https://github.com/jbkoch/Curbeloetal_SWD.
results
Field Study
A total of 2,053 D. suzukii (female = 1,070; male = 983) were collected during 140 trap deployments at 14 field sites on the eastern flank of Mauna Loa from March to August 2016. In forested and non-forested sites, we detected a total of 1,596 (mean = 22.8) and 457 (mean = 6.52) D. suzukii, respectively (Figure 2). The mean deployment time for all traps is 15.1 days (minimum = 4, maximum = 36). In total, we collected 3,350 mL of V. reticulatum berries across six of the non-forested field sites over the course of 35 sampling events. From the sites collected, HAVO2b had the greatest number of individuals emerge relative to the number of berries observed (16 individuals emerged; 1.88% emergence per mL) (Table 2). No D. suzukii emerged from the collected berries in HAVO7b, HAVO6b, and HAVO1b. In addition to D. suzukii, nine Braconidae (Hymenoptera) and 25 Lepidoptera [likely Carposina inscripta (Walsingham 1907) (Lepidoptera: Carposinidae)] were documented to emerge from ‘ōhelo berries across all field sites and sampling events.
D. suzukii Abundance Model
The initial global model was highly over-dispersed at ĉ = 16.68. After iterative examination of β s following the removal of significant observation outliers, we elected to remove 12 observations from the global GLM. Removal of the outliers resulted in an overdispersion estimate of ĉ = 11.33 (n = 128, 91% of data retained for analysis). Thus, we used the quasi-Poisson link function in the subsequent global model. Based on QAICc and wi, only one model was identified as the top model. The top model included altitude, Julian day, and habitat to best explain D. suzukii abundance data [Abundance ~ 3.15 + 0.0009 (Altitude) + 0.006(Julian day)+ 0.90(Habitat)] (Table 3). Relative to the top model (wi = 0.64), the subsequent models exhibited a ΔQAICc > 2, with a mean wi = 0.03 (12 models, not including null model) (Table 2). Our model estimates that D. suzukii abundance decreases across an altitude gradient by 0.0009 flies (95% CL = 0.001, 0.0006) for each 1 m increase in altitude (Figure 3A). Furthermore, for each increase in Julian day, we estimate a 0.006 increase (95% CL = 0.002, [End Page 517] 0.010) in the abundance for D. suzukii flies detected in a Haviland trap (Figure 2). Finally, our model predicts a decrease of 0.90 flies (95% CL = 1.22, 0.573) in non-forested habitats relative to forested habitats (Figure 3B).
Mean (±SE) D. suzukii abundance across trap locations relative to time (Julian day) separated by habitat type in Hawai‘i Volcanoes National Park.
Ōhelo (Vaccinium reticulatum) Emergence Incidence of Drosophila suzukii on Mauna Loa, Hawai‘i
discussion
Our study characterizes D. suzukii host-use and population abundance across an altitude gradient of 1315 m on Mauna Loa, Hawai‘i over a brief four month period. We found that D. suzukii readily uses ‘ōhelo berries as a host for reproductive activities in habitats composed primarily of native plant species. Furthermore, our global model found that D. suzukii abundance decreases across an altitude gradient on Mauna Loa and is more abundant in forested habitats compared to non-forested habitats. Finally, advancing [End Page 518] Julian day was a significant predictor of D. suzukii abundance but not Haviland trap deployment time.
Drosophila suzukii abundances across an (A) altitude gradient and (B) habitat type. In (A), forested habitats are represented by black circles and non-forested habitats are represented by gray squares.
Top Competing Models Based on Quasi-Akaike’s Information Criterion Adjusted for Small Sample Sizes (QAICc) and the Null Model are Shown for Models Examining Factors that Influence the Abundance of Drosophila suzukii on Mauna Loa, Hawai‘i
We documented the highest emergence percentage of D. suzukii in the non-forested habitat of HAVO2b, where we surveyed 850 mL of fruit. In general, we found that the mid-altitude habitats had the highest D. suzukii emergence rate (Table 2), whereas the highest and lowest sites yielded no emergence despite high detection in forested field sites (Figure 3). Given our ability to detect D. suzukii across all sites, as well as the absence of ‘ōhelo in forested sites in our study, it is possible that D. suzukii is able to oviposit into non-fruits when a suitable fruit host is not available (Stockton et al. 2019a,b). Along with other studies, our study suggests that their ability to persist in a novel environment is not limited to the availability of a hospitable fruit host (Stockton et al. 2019a,b). In addition to ‘ōhelo, we collected berries of pukiawe (Leptecophylla tameiameiae) and kukaenene (‘aiakanene) (Coprosma ernodeoides) when they [End Page 519] were available on our field sites. However, we did not collect them in a systematic way and could not present data to support this observation. However, from the non-‘ōhelo berries we collected, we found no D. suzukii to emerge, or any other arthropod. In addition to D. suzukii, we observed Braconidae and 16 Lepidoptera larvae to emerge from ‘ōhelo berries. The vast majority of Braconidae in Hawai‘i are non-native (Peck et al. 2008), thus we have likely detected a non-native Braconidae using ‘ōhelo for its reproductive activities. The Lepidoptera detected in our study are likely Carposina inscripta (Walsingham, 1907) (Lepidoptera: Carposinidae), as the larvae are known to feed on ‘ōhelo berries (Zimmerman 1978). Future work should characterize ‘ōhelo berry host use across non-native and native insects.
D. suzukii abundance was found to decrease across an altitude in our study. Increasing altitude is associated with cooler temperatures (Table 1), which may be a limiting factor of population growth. Cooler temperatures are associated with longer gestation periods in D. suzukii which ultimately lead to increased melanization and body size (Shearer et al. 2016). However, from an evolutionary perspective, D. suzukii is adapted to persist in temperate climates (Ometto et al. 2013). Its ability to successfully invade temperate and montane-temperate regions of North America and Europe is likely due to its Asian origin since the end of the Miocene (~9–6 Mya) (Ometto et al. 2013). In addition to cooler temperatures, high altitude populations of Hawaiian D. suzukii are exposed to lower air density. Low air density may facilitate lower survival as high-altitude populations would need to develop larger wing areas relative to body size to efficiently sustain flight, which could impact their ability to disperse (Dudley 2002). Middle to low altitude locations that exhibit mesic temperatures might facilitate larger populations of D. suzukii compared to higher altitude environments. While high altitude habitats are suitable for D. suzukii, the middle to low altitude habitats may be more favorable in supporting large populations. Thus, even though D. suzukii is predisposed to succeed in montane temperate climates (Ometto et al. 2013), it appears that middle to low elevation habitats that are associated with warmer temperatures are most ideal for supporting large D. suzukii populations in Hawai‘i (Mitsui et al. 2010, Iglesias et al. 2014, Stockton et al. 2018, 2019a,b, 2020). In fact, population genetic analysis demonstrates that D. suzukii can move across elevation gradients at distances of ~1 km from their natal habitats during their adult life time (Tait et al. 2018).
Our study found that habitat-type was a strong predictor of D. suzukii abundance. Specifically, we detected greater abundances in forested habitats relative to non-forested habitats. We did not detect any suitable host plants in our plant surveys of the forested habitat. Forested habitats in our study were primarily composed of plant species that did not produce a fruit host for D. suzukii such as ‘ōhi‘a (Metrosideros polymorpha) and koa (Acacia koa). However, the forested habitats we surveyed were close to the non-forested habitat (500 m–1000 km). Thus, it is likely that D. suzukii individuals are moving across the elevation gradient and habitat types on Mauna Loa in response to diverse ecological factors (e.g., host availability, temperature, etc.) (Mitsui et al. 2010, Tait et al. 2018). A recent study by Koch et al. (2020) found no evidence for genetic structuring across D. suzukii populations within islands, supporting the hypothesis that D. suzukii is dispersing across diverse habitats (Leblanc et al. 2009, 2013, Mitsui et al. 2010, Tait et al. 2018, Tonina et al. 2018). Middle to low altitude habitats are dominated by higher abundance and diversity of native and nonnative plants in Hawai‘i compared to higher altitudes (Price et al. 2012). Thus, an alternative hypothesis for larger D. suzukii population sizes in middle- and low-altitude habitats is the availability of non-native hosts (Tonina et al. 2018). Across croplands and forested areas, D. suzukii are documented to disperse away from their natal sites in search of fruit hosts using both passive sampling techniques and genetic analysis (Mitsui et al. 2010, Tait et al. 2018, Tonina et al. 2018). A study by Tonina et al. (2018) demonstrated that D. suzukii will use forest margins more readily [End Page 520] when host fruits are not available in agricultural landscapes.
The rapid spread of D. suzukii across habitats composed of native and non-native species in Hawai‘i is creating novel species interactions (Leblanc et al. 2009, 2013). While endemic Hawaiian Drosophila are found predominantly in native ecosystems, they are also found in spaces occupied by invasive strawberry guava (Psidium cattleyanum) and plantation forests near the native forests (Leblanc et al. 2013). D. suzukii is known to actively use strawberry guava as a host (Follett et al. 2014). Thus, we suspect that D. suzukii may be interacting with endemic, and potentially endangered native Drosophila species. However, D. suzukii has been demonstrated to be a poor competitor with D. melanogaster based on egg laying and emergence success (Shaw et al. 2018). Thus, determining how D. suzukii responds to competition with native and non-native insects among host-fruits in Hawai‘i is ripe for future research. Interestingly, while most introduced Drosophila species observed were found in disturbed habitats, many were abundant in even the most pristine native forests (Leblanc et al. 2013). Our study implies that D. suzukii is straying away from habitats rich in non-native plants and finding suitable hosts in ecosystems dominated by native and endemic plant species (Mitsui et al. 2010, Tonina et al. 2018). Plant dispersal can be negatively affected by infestation, particularly because D. suzukii prefers to oviposit in fresh, not fallen or rotting, fruit (Ometto et al. 2013). Many plant species have experienced premature fruit falling or splitting due to D. suzukii infestation (Walsh et al. 2011), which would reduce the viability and dispersal capability of the seeds. Premature fruit falling also has the potential to decrease the attractiveness of the fruit to dispersers such as birds. Birds have been documented to prefer fruit that has not been infested with insects (Pairon et al. 2006). D. suzukii could be limiting the dispersal of endangered or at-risk endemic Hawaiian plants, which could have consequences on native fauna populations. However, further research is needed to address these conservation concerns.
In conclusion, D. suzukii is an established invasive insect in the Hawaiian archipelago. It readily uses ‘ōhelo for its reproductive activities and is likely moving across the landscapes to colonize different environments. It does not appear to be limited to habitats composed of native plants, as it is also found in habitats dominated by non-native plant species, specifically the highly invasive guava and strawberry guava (Koch et al. 2020). D. suzukii is most abundant at low to middle altitude field sites on Mauna Loa, where the average annual temperature is 18.8 °C (Table 1). However, they are also readily found at high altitude field sites, particularly in forested areas. A more replicated study of D. suzukii host use would be appropriate to determine whether the species competes with native arthropods in their use of native plant species. Furthermore, a year-round study of D. suzukii population dynamics would provide insight into whether the species is at all limited to different microclimates and habitat-types of Hawai‘i.
Current address: School of Life Sciences, University of Nevada-Las Vegas, 4505 S. Parkway, Las Vegas, NV, 89154 USA.
Current address: U.S. Department of Agriculture - Agricultural Research Service - Pollinating Insect - Biology, Management, Systematics Research Unit, Utah State University, Department of Biology, UMC5310, Logan, UT 84322, USA.
acknowledgments
Mahalo to Dr. Rhonda Loh and Sierra McDaniel for supporting our research in HAVO, and the Price Lab for comments that greatly improved this work. Our study of D. suzukii was made possible by a permit from HAVO (HAVO-2016-SCI-015).