Eleotris (Teleostei: Eleotridae) from Indonesia with Description of Three New
Species Within the ‘melanosoma’ Neuromast Pattern Group
The species of Eleotris from Indonesia are reviewed and compared to the known species described from the area. Nine species are recognized including three new species in the ‘ melanosoma’ neuromast pattern group. These are described using genetic and morpho-meristic approaches. The new species differ by a high percentage of genetic divergence in partial COI gene (652 bp) and by several characters including the number of pectoral fin rays, the number of scales in lateral, predorsal, forward and zigzag series. The main characteristics of the other known species in the area in the ‘melanosoma’ group, Eleotris melanosoma Bleeker, 1853 and Eleotris macrolepis (Bleeker, 1875), both belonging to this group, are given for comparison. A key for Eleotris species from Indonesia is provided.
Eleotris, cryptic species, Indonesia
Among amphidromous fishes, the genus Eleotris (Teleostei: Eleotridae) is one of the most common in the Indo-Pacific area. It is found in the lower and medium parts of insular freshwater streams. The species spawn in freshwater and, after hatching, larvae drift downstream to the sea where they undergo a 3–4 months’ planktonic phase (Mennesson et al. 2015). After this marine phase, individuals return to rivers to grow and reproduce ( McDowall 2007, Maeda et al. 2007). In these rivers, Eleotris is a sit-and-wait predator, a carnivorous species distributed primarily in freshwater, even if several species are more estuarine (Maeda and Tachihara 2004). They generally live close to the riverbank where the current is slow, or in the lentic zones below stones or dead wood (Nordlie 1981, Keith et al. 2010). Adult Eleotris species are not, in most places, targeted as a food resource; they are however targeted for human consumption at the post-larval stage as they recruit back to rivers (Perrone and Vieira 1991, Pezold and Cage 2002, Mennesson et al. 2019).
It is well known that field identification of Eleotris species is difficult due to the lack of useful meristic characters and because all the species are generally brown and tend to look alike. Nevertheless, several authors have [End Page 469] shown that the presence and disposition of cephalic sensory papillae rows on the operculum and under the eyes are diagnostic characters (Akihito 1967, Miller 1998, Pezold and Cage 2002, Mennesson et al. 2019). For the Pacific species, Akihito (1967), studying the Eleotris from Japan, was the first to propose four different patterns of row arrangement (see Materials and Methods) corresponding to the species he studied (patterns ‘2’ for E. oxycephala Temminck & Schlegel, 1845; ‘2.4’ for E. acanthopomus Bleeker, 1853, E. sandwicensis Vaillant & Sauvage, 1875 and E. mauritiana Bennett, 1832; ‘2.3.4’ for E. melanosoma Bleeker, 1853; and ‘2.4.6’ for E. fusca (Bloch & Schneider, 1801)). Later, Mennesson et al. (2019) demonstrated that the Eleotris phylogeny in the Indo-Pacific area reflects the morphology of the opercular papillae and added a fifth pattern (‘2.4.5.6’: Eleotris eigenmanni Popta, 1921). Finally, five different patterns of row arrangement have been used by Mennesson and Keith (2020) to study the Indian Ocean Eleotris species. These patterns are indeed of major importance to distinguish species (see Material and Methods), as difference in squamation and number of rays in the pectoral fins.
The genus Eleotris has never been reviewed in Indonesia and, among the four biodiversity hotspots identified in South East Asia (Myers et al. 2000), the two Indonesian ones (Sundaland and Wallacea) are currently the most threatened ( Hubert et al. 2015). The threat of the ichthyodiversity in these hotspots is of great concern and the taxonomic knowledge is still incomplete. Unfortunately, filling this gap is currently jeopardized by the fast degradation of the Indonesian natural habitats (Hubert et al. 2015 ). So far, nearly 1,200 species of freshwater fishes have been reported from Indonesian inland waters and the rate of species discovery is still high, as tens of species have been described from Indonesia during the last years (Hubert et al. 2015), and particularly during the collaborative work between the Institute for Research and Development (IRD), the Indonesian Institute of Sciences (LIPI) and the National Museum of Natural History of Paris (MNHN). This work has led to expeditions to remote areas (Sulawesi, Sumatra, Java, Kalimantan, Lombok, Bali, Ambon, Halmahera and Ceram) and has resulted in the collection of many specimens and the discovery of several new species (Keith et al. 2012a , b , Pouyaud et al. 2012, Keith et al. 2014a , b , Larson et al. 2014, Hoese et al. 2015, Keith et al. 2015a , b , Keith and Hadiaty 2015, Keith et al. 2017, Keith et al. 2018, Delrieu-Trottin et al. 2020, Sholihah et al. 2020 ).
The purpose of this paper is to review the Eleotris species found in Indonesia, using, when possible, genetic and morphometric approaches. A key for the species of the area is also provided. This work will complement that of Mennesson and Keith (2020) who reviewed the Eleotris of the Indian Ocean and thus will provide a basis for determining the species of the Pacific Ocean.
materials and methods
DNA Extraction and Amplification
A total of 117 Eleotris specimens were used for the barcode analysis (see Table 1A). A fin clip or a muscle biopsy was taken for each specimen and fixed in a 96% ethanol solution. Then, voucher specimens were preserved in a 5% formalin solution.
Tissue was used to extract total genomic DNA using the Macherey & Nagel Nucleo Spin® Tissue kits following the manufacturer’s instructions on an Eppendorf EpMotion 5075.
The DNA barcode fragment of the cytochrome oxidase I (COI) mitochondrial gene was amplified using primers FishF1-5′TCAACCAACCACAAAGACATTGGCA C3′ and FishR1-5′ACTTCAGGGTGAC CGAAGAATCAGAA3′ (Ward et al. 2005). All PCRs were performed on Biometra thermocyclers in a 25 μl volume of 5% of DMSO, 5 μg of bovine serum albumin, 300 μM of each dNTP, 0.3 μM of Taq DNA polymerase from Qiagen, 2.5 μl of the corresponding buffer, and 1.7 pM of each of the two primers. After a 2-min denaturation at 94 °C, the PCR ran 50 cycles of 25 s at 94 °C, 25 s at 52 °C and 1 min at 72 °C, with a [End Page 470]
Specimens Used for the DNA Barcode Analysis (Names, Sequences and Barcode
[End Page 473]
3-min terminal elongation. Purification and Sanger sequencing of PCR products were performed by Eurofins (http://www.eurofins.fr) using the same forward and reverse PCR primers. Chromatograms were assembled and edited using Geneious 8.1.5. All the sequences were aligned with MAFFT Alignment (implemented in Geneious). The percentage of divergence between sequences was calculated on Geneious 8.1.5. The translation into amino acids was checked for the partial fragment of COI gene, using the vertebrate mitochondrial genetic code. After translation, one or two bases were discarded at the beginning and the at end of the sequences and as a result all the sequences in the alignment started and ended with a codon. All the sequences have been deposited in the barcode of life data system (www.boldsystems.org; dx.doi.org/10.5883/DS-ELEO ) as well as GenBank (accession numbers accessible through BOLD).
Phylogenetic relationships within Indonesian specimens were inferred using the Maximum Likelihood (ML) algorithm as implemented in phyml 3.0.1 (Guindon and Gascuel 2003). The optimization of the ML tree topology was conducted using the BEST tree rearrangement option combining both Nearest-Neighbor Interchange (NNI) and Subtree Pruning and Regrafting (SPR). The best-fit ML substitution model was selected among 88 models according to the Bayesian Information Criterion (BIC) as implemented in jmodeltest 2.1.7 (Darriba et al. 2012). Delineation of mitochondrial lineages with independent evolutionary dynamics was performed using the Refined Single Linkage (RESL) algorithm as implemented in BOLD and each cluster of sequence was assigned to a Barcode Index Number (BIN) in BOLD (Ratnasingham and Hebert 2013).
A second phylogenetic tree was built with specimens collected outside Indonesia to show the wider distribution known of the species studied. This tree was performed on the COI gene (576 bp) alignment using Bayesian inference (MrBayes v.3.2; Ronquist et al. 2012). Three models, corresponding to the three-codon positions, computed in PartitionFinder (Lanfear et al. 2012) (1st position, HKY+G model; 2nd position, SYM + I model; 3rd position, F81 model) were run for 10 million generations, sampling every 200 generations with two independent runs to access convergence. Run convergence was checked using TRACER v.1.6.0 (Rambaut and Drummond 2007). Trees were summarised using the 50% majority rule method after discarding the first 25% of the sample as burnin and visualised using FigTree v.1.4.2 ( Rambaut 2007). The sequence of Bunaka gyrinoides (Eleotridae) was included as out-group.
Morpho-Meristic Study
Method follows Akihito (1967) and was adapted for zigzag and transverse scales series as explained below by Mennesson (2016) . Specimens were measured with a dial calliper to the nearest tenth of a millimetre. All counts were taken from the right side. The size is given as standard length (SL).
Scale and fin ray counts are reported as: A, anal fin elements (includes flexible spine and segmented rays); D, dorsal fins (D1, first dorsal fin spines; D2, second dorsal fin elements); P, pectoral fin rays; C, caudal fin rays (only branched rays are reported); LS, scales in lateral series counted from upper pectoral fin base, or anteriormost scale along lateral midline, to central hypural base; PD, predorsal midline scales counted from scale directly anterior to first dorsal fin insertion to the anteriormost scale; TRB, transverse series backward, refers to scales counted from the first scale anterior to second dorsal fin origin, in a diagonal manner, posteriorly and ventrally to the anal fin base or ventralmost scale; TRF, transverse series forward, refers to scales counted from the first scale anterior to second dorsal fin origin, in a diagonal manner, anteriorly and ventrally to the centre of abdomen or ventralmost scale; ZZ, zigzag series, refers to scales on the narrowest region of the caudal peduncle counted from the dorsalmost scale to the ventralmost scale in a zigzag (alternating) manner. Finally, the cephalic neuromast distribution patterns were examined and illustrated with the aid of a dissecting microscope and camera lucida. [End Page 474]
The five different cephalic neuromast patterns in Eleotris.
Eleotris species are mainly distinguished by the superficial neuromast patterns of the head (Akihito 1967). Cephalic neuromast patterns are described using terminology developed by Sanzo (1911) with modifications employed by Miller and Wongrat (1991) and Pezold and Cage (2002). Transverse opercular rows are labelled ot. Upper and lower longitudinal rows on the operculum are labelled os and oi respectively. Transverse suborbital rows are designated with Arabic numbers and major horizontal rows on the cheek are indicated with the letters b and d. To simplify references to the particular transverse suborbital rows crossing row d, a formula of row numbers separated by periods is used (Mennesson et al. 2019). For example, if rows 2, 4 and 6 cross row d, this condition is represented by the formula ‘2.4.6’.
Five different patterns have been noted by Mennesson et al. (2019) : pattern ‘2’ for the ‘Eleotris oxycephala group’, ‘2.4’ for the ‘ Eleotris acanthopomus group’, ‘2.3.4’ for the ‘Eleotris melanosoma group’, ‘2.4.6’ for the ‘Eleotris fuscagroup’, and ‘2.4.5.6’ for E. eigenmanni (Figure 1).
Types and other specimens were examined from museum collections (MNHN: Muséum national d’Histoire naturelle, Paris; RMNH: Rijksmuseum van Natuurlijke Historie, Leiden; SMNS: Staatliches Museum für Naturkunde, Stuttgart; ZMH: Zoological Museum Hamburg; BMNH: Natural History Museum, London; CAS-SU: California Academy of Sciences (San Francisco), Stanford University (Palo Alto, California); WAM: Western Australian Museum, Perth, Western Australia; MZB (Museum Zoologicum Bogoriense, Bogor, Indonesia); SMF: Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt; ZMH: Zoological Museum Hamburg; USNM: National Museum of Natural History, Smithsonian Institution, Washington D.C.).
In total 165 Eleotris specimens, including types, were studied (see ‘Comparative material’ at the end of the paper). They were ranked here according to the superficial neuromast patterns of their head, the first and major criterion to distinguish species groups.
results and discussion
DNA Barcode Analysis
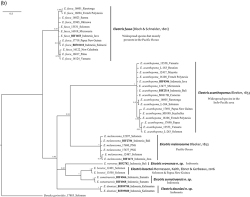
A total of 652 base pairs were amplified for the COI gene from the Eleotris individuals and deposited in BOLD (www.boldsystems.org; DS-ELEO:doi) as well as GenBank (accession numbers accessible through BOLD). The most likely substitution model selected by jmodeltest was TPM1uf + G. The ML tree [End Page 475] (Figure 2A ) allowed us to delimit seven lineages among six species, each corresponding to a distinct mitochondrial lineage as evidenced by the RESL algorithm. The tree presents two branches; the first one (I) is composed of Eleotris acanthopomus (BOLD: AAI9151) and the second one (II) is composed of 3 sub-branches. The first one (A) is composed of Eleotris fusca (BOLD: AAF0108). For the second one (B), two lineages are noticed within E. melanosoma , a cluster observed only in Java (BOLD: AAF0109) and a second widely distributed in Java, Sumatra and Bali (BOLD: AAK9481). The third one (C) is composed of three candidate species with the neuromast pattern ‘2.3.4’ (i.e., ‘melanosoma group’; group defined by Mennesson et al. 2019): Eleotris douniasi n. sp . (BOLD: AEA5081), Eleotris woworae n. sp. (BOLD: ACQ5280) and Eleotris sumatraensis n. sp. (BOLD: ADF2921).
Most likely ML tree inferred using the TPM1uf+G model (ln L = 2414.6323, G = 0.17, f(A) = 0.2433, f(C) = 0.3077, f(G) = 0.1819, f(T) = 0.2670, AC = GT = 1.0, AT=CG=19.7855, AG=CT=2.3830).
A simplified pairwise distances matrix was done (Table 1B) to show the percentage of divergence within and between species and their distribution within the Indo-Pacific area is observable in Figure 2B .
Morpho-Meristic Study
Regardless of the genetic results, after examination and measurement of the 165 studied specimens (including type specimens), the morphological and meristic data showed that nine species of Eleotris were present in Indonesia, including 3 candidate species with the neuromast pattern ‘2.3.4’ (melanosoma group), the same ones highlighted by the molecular data (Tables 2–4). This correlation between morpho-meristic and molecular data allows us to conclude that they are new species. These species differ by several characters including the superficial neuromast [End Page 476] patterns of the head, the number of pectoral fin rays, the number of scales in lateral, predorsal, forward and zigzag series.
Pairwise Mean Distances Matrix
Bayesian tree of the COI gene fragment (576 bp) for sequences specimens of Eleotris. Numbers on nodes represent posterior probabilities.
Among the Indonesian nine species found, one has the neuromast pattern ‘2.4’ (E. acanthopomus Bleeker, 1853); five have the pattern ‘2.3.4’ (E. melanosoma Bleeker, 1853, E. macrolepis (Bleeker, 1875) and the three new species, E. woworae n. sp., E. sumatraensis n. sp. and E. douniasi n. sp .); two have the pattern ‘2.4.6’ (E. fusca (Bloch & Schneider, 1801) and E. macrocephala (Bleeker, [End Page 477]
Scale Counts in Eleotris with a ‘2.3.4’ Pattern in Indonesia
[End Page 478]
Pectoral Ray Counts in Eleotris with a ‘2.3.4’ Pattern in Indonesia
Selected Morphometrics in Eleotris with a ‘2.3.4’ Pattern in Indonesia
1857)); and one has the pattern ‘2.4.5.6’ (E. eigenmanni Popta, 1921). The three new species are described thereafter.
As already shown by Mennesson and Keith (2020) for the Indian Ocean, where they recently described two new species from the ‘melanosoma group’ ( i.e., pattern ‘2.3.4’) the cryptic species in Indonesia are also from this group, which finally remained really poorly known. Numerous specimens registered as ‘E. melanosoma ’ in many collections are in fact probably not this species and there are potentially still new species left to discover in this group.
Our current study also follows Mennesson and Keith (2020) who re-elevated Eleotris macrolepis (Bleeker, 1875), also from the ‘melanosoma group’, to full species status based on examination of the two type specimens only. Unfortunately, we prospected the type locality (Ambon Island, Molucca, Indonesia) but failed to find this species. E. macrolepis differs from the other species present in the area in having 15 pectoral rays versus 16–20, larger scales and a larger jaw.
The main characteristics of E. melanosoma and E. macrolepis are given below.
Eleotris melanosoma Bleeker, 1853
Figures 1–3; Tables 2–4
Culius insulindicus Bleeker, 1875
Eleotris pseudacanthopomus Bleeker, 1853
Eleotris melanura Bleeker, 1849
Eleotris hainanensis Chen, 1933 [End Page 480]
Material examined
Syntypes: in RMNH 4815, 3 males (63–84 mm) from Wahai, Ceram, Indonesia.
Other material (N = 26)
Culius insulindicus Bleeker, 1875: syntypes in RMNH 4804, 2 males (62–84 mm) from Sumatra, Indonesia. Eleotris pseudacanthopomus Bleeker, 1853: holotype, male (63 mm) from Western Sumatra, Indonesia (SMNS 10595). Eleotris melanura Bleeker, 1849: holotype (36 mm) from southern Java, Indonesia (in RMNH 5182).
Twenty-two other specimens of E. melanosoma from seven different localities (see ‘Comparative material’).
Diagnosis
The species is distinguished by second, third and fourth suborbital free neuromast rows on cheek extending ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern), 16–17 pectoral fins rays, 45–52 scales in lateral series and 37–44 scales in predorsal series.
Description
Scale and rays counts in Eleotris melanosoma and related species are given in Tables 2 and 3, selected morpho-meristics in Table 4.
The body is elongate. The body depth at anus is 18–21% SL and the caudal peduncle depth is 11–16% SL. Predorsal length 44–49% SL and preanal length 62–68% SL. Size up to 90 mm SL.
The head (32–37% SL) is depressed, the snout is pointed. Eyes high on head. The mouth is large, as jaw length is 11–14% SL, and oblique, the posterior margin of upper jaw reaches the vertical through the first third of the eye. Upper and lower jaws with multiple rows of small teeth, larger on outer row; a few caniniform teeth in anterior position. Tubular anterior nares overhanging two third upper lip, posterior nares open pits.
Dorsal fins VI-I,8; D1 separate from and smaller than D2; spines not elongate. Anal fin I,8 and directly opposite to second dorsal fin. Pelvic fins separate, I,5. Pectoral fins 16–17. Caudal fin pointed with 15 branched rays.
Cycloid scales on top of head, nape, pectoral fin base, pre-pelvic region and abdomen. Cheek and operculum mostly naked. Ctenoid scales covering flanks. Scales 45–52 in lateral series; 37–44 in predorsal series, 13–17 in transverse back series, 20–26 in transverse forward series and 11–13 in zigzag series.
Gill opening is moderately broad, extending to below the preoperculum. Outer gill rakers on the first gill arch 12–13, they are rods with spines on their inner side.
Cephalic lateralis: adults with 5 transverse suborbital free neuromast rows of which second, third and fourth on cheek extend ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern) (Figure 1D ).
Urogenital papilla elongated and tapered in males, rounded in females.
Colour in life (Figure 3)
Male and female similar. Two different patterns observed in several localities. The first one with top of head, top of the eye and back yellow-brown. Lips brown with several small beige blotches. Cheeks and operculum with star-like patchy beige and brown areas. Lateral part of body brown, with several rows of beige to whitish scales. Abdomen and gular region brown. First dorsal fin with 2 large greyish bands alternating with 2 translucent bands; rays with yellow to orange alternating with black wavy spotted rows. Second dorsal, anal and pelvic fins with yellow to orange alternating with black wavy horizontal stripes. Caudal fin dark brown with vertical rows of beige dots; several beige to whitish patches of scales. Pectoral fins yellowish and translucent, with a white band at the base and a black blotch at the upper part of this base. The second pattern is quite similar except that the body is more uniformly brownish on the flanks, without patches of beige areas.
Colour in preservation
Head, preoperculum and body brownish. Dorsal, pectorals, anal and caudal fins yellowish to brownish and translucent. Pelvic fins and belly yellowish. [End Page 481]
Eleotris melanosoma, from Bali, Indonesia (58 mm SL); BIF 2168
Comparison
E. melanosoma differs from the other species sequenced with a ‘2.3.4’ pattern and present in the area by displaying reciprocal monophyly from its closest relatives and high TPM1uf + G genetic distances to its relatives at COI gene (10.8% to 14.1% of divergence). Moreover, it differs from E. macrolepis, from E. woworae (this paper) and from E. douniasi (this paper) in having 16–17 pectoral rays versus 15, 19 and 19–20 respectively. It differs from E. sumatraensis (this paper) in having 37–44 scales in predorsal series versus 31–33, and 11–13 scales in zigzag series versus 9–11.
Distribution
E. melanosoma is not present in West Indian Ocean but restricted to Pacific Ocean ( Mennesson et al. 2019). Known from Sumatra, Java, Bali, Ceram, Philippines, Taiwan, Papua, Papua New Guinea, Solomon Islands and New Caledonia. In Indonesia, it was found in sympatry with E. fusca and E. acanthopomus (Java), with E. fusca and E. sumatraensis (Sumatra) and with E. fusca and E. woworae (Bali). The species could exist in other regions.
Ecology
The species is thought to be amphidromous. It is found in the lower part of the rivers, from the estuary to 30 m in elevation. It lives in river with mixed sandy-muddy bottoms and scarce patches of riparian vegetation under tidal influence or in running water, with mixed sandy-rocky bottoms. It is carnivorous and eats small fishes (as Gobiidae juveniles and post-larvae) and shrimps ( Caridina and Macrobrachium species).
Eleotris macrolepis (Bleeker, 1875)
Figures 1, 2, 4; Tables 2–4
Material examined
Type material
Syntypes: RMNH 4759, 2 males (51–57 mm SL) from Ambon, Indonesia.
Diagnosis
The species is distinguished by second, third and fourth suborbital free neuromast rows on cheek extending ventrally past horizontal row d (‘2.3.4’ i.e., ‘ melanosoma group’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern), 15 pectoral fins rays, 42–43 scales in lateral series and 16–19 in transverse forward series, and a large mouth (16–17% SL).
Description
Based only on the two male syntypes. Scale and rays counts in Eleotris macrolepis and related species are given in Tables 2 and 3, selected morpho-meristics in Table 4.
The body is elongate but the body depth at anus is 22–23% SL and the caudal peduncle depth is 14–16% SL. Predorsal length 44–49% SL and preanal length 65–68% SL.
The head (33–34% SL) is depressed, the snout is pointed. Eyes high on head. The mouth is very large (jaw is 16–17% SL), and oblique, the posterior margin of upper jaw reaches a vertical nearly through the posterior [End Page 482] edge of the eye. Upper and lower jaws with multiple rows of small teeth. Tubular anterior nares overhanging half upper lip, posterior nares open pits.
Dorsal fins VI-I,8-9; D1 separate from D2; spines not elongate. Anal fin I,8 and directly opposite to second dorsal fin. Pelvic fins separate, I,5. Pectoral fins 15. Caudal fin pointed with 15-branched rays.
Cycloid scales on top of head, nape, pectoral-fin base, pre-pelvic region, and abdomen. Operculum with small-embedded cycloid scales dorsally, cheek naked. Ctenoid scales covering flanks. Scales in lateral series 42–43; in predorsal series 31–35, in transverse back series 13, in transverse forward series 18–19 and in zigzag series 11.
Gill opening is moderately broad, extending to below the preoperculum.
Cephalic lateralis: adults with 5 transverse suborbital free neuromast rows of which second, third and fourth on cheek extend ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern) (Figure 1D ).
Urogenital papilla elongated and tapered in males.
Colour in life
Unknown
Colour in preservation (Figure 4)
Head, preoperculum and body brownish. Dorsal, pectorals, anal and caudal fins yellowish to brownish and translucent. Pelvic fins and belly whitish.
Comparison
E. macrolepis differs from the other species present in the area in having 15 pectoral rays versus 16–20 and a larger jaw (16–17% SL versus 9–14% SL).
Distribution
Currently known only from Ambon (Indonesia).
Ecology
Unknown.
Eleotris woworae sp. nov. Keith, Mennesson, Sauri, Hubert
Figures 1, 2, 5; Tables 2–4
Material examined
Type material
Holotype: MZB.25313, male (86.4 mm SL), Kab Kelungkung, Tukad Unda River, Bali, Indonesia, 22 Apr. 2014, Hubert et al. coll., BIF 2782.
Other material (N = 52)
For the other species studied and compared in the 2.3.4 ‘melanosoma group’ pattern, see ‘Comparative material’.
Eleotris macrolepis, syntype RMNH 4759, from Ambon, Indonesia (unlabelled specimen, 51 mm SL)
[End Page 483]
Diagnosis
The species is distinguished by second, third and fourth suborbital free neuromast rows on cheek extending ventrally past horizontal row d (‘2.3.4’ ‘melanosoma group’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern), 19 pectoral fins rays, 55 scales in lateral series and a high body depth at anus (22% SL). A single mitochondrial lineage (BOLD: ACQ5280).
Description
Based only on one specimen, the holotype. Scale and rays counts in Eleotris and related species are given in Tables 2 and 3, selected morpho-meristics in Table 4.
The body is elongate. The body depth at anus is 22% SL and the caudal peduncle depth is 15% SL. Predorsal length 47% SL and preanal length 67% SL.
The head (35% SL) is depressed and broad, the snout is more or less rounded. Eyes high on head. The mouth is large, as jaw length is 13% SL and oblique, the posterior margin of upper jaw reaches the vertical through the first fourth of the eye. Upper and lower jaws with multiple rows of small teeth, larger on outer row; a few caniniform teeth in posterior position. Tubular anterior nares overhanging half upper lip, posterior nares open pits.
Dorsal fins VI-I,8; D1 separate from and smaller than D2; spines not elongate. Anal fin I,8 and directly opposite to second dorsal fin. Pelvic fins separate, I,5. Pectoral fins 19. Caudal fin pointed with 15-branched rays.
Cycloid scales on top of head, nape, pectoral-fin base, pre-pelvic region and abdomen. Operculum with small-embedded cycloid scales dorsally, cheek naked. Ctenoid scales covering flanks. Scales in lateral series 55; in predorsal series 38, in transverse back series 16, in transverse forward series 22 and in zigzag series 13.
Gill opening is moderately broad, extending to below the preoperculum. Outer gill rakers on the first gill arch, they are short, wide at the base and tooth-shaped.
Cephalic lateralis: adults with 5 transverse suborbital free neuromast rows of which second, third and fourth on cheek extend ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed pattern’) (Figure 1D ).
Urogenital papilla elongated and tapered in the single male specimen.
Colour in life (Figure 5)
Head and preoperculum dark brown or black with several yellowish patches. Flanks light brown. Abdomen and gular region greyish to whitish slightly punctuated with small dark spots. First dorsal fin with three large dark bands alternating with two thin orange ones. Second dorsal, and anal fins with five brownish wavy spotted rows alternating with five yellow ones. Pelvic fins translucent. Caudal fin dark brown to brown, without spots anteriorly, each ray alternating yellow and black spots in 12–13 rows. Pectoral fins predominantly yellowish and translucent with sparse black spotting.
Colour in preservation
Head and preoperculum dark brown or black with several lighter patches. Flanks brownish. Abdomen and gular region greyish to whitish tightly punctuated with small dark spots. First dorsal fin with three large dark bands alternating with two thin white ones. Second dorsal and anal fins with five brownish wavy spotted rows alternating with five white ones. Pelvic fins whitish. Caudal fin dark brown alternating white and black spots in 12–13 rows. Pectoral fins predominantly whitish with sparse black spotting.
Etymology
The new species is named woworae in dedication to Daisy Wowor from the Division of Zoology of the Indonesian Institute of Sciences (LIPI), Cibinong, who helped us to collect freshwater fishes all around Indonesia.
Comparison
E. woworae differs from the other species sequenced with a ‘2.3.4’ pattern and present in the area by displaying reciprocal monophyly from its closest relatives and high TPM1uf+G [End Page 484] genetic distances to its relatives at COI gene (9.4% to 16.1% of divergence). Moreover, it differs from the other species of the area except E. douniasi (this paper) in having 19 rays in pectoral fins versus 16–17. It differs from E. douniasi in having more scales in lateral series (55 versus 41–44).
Eleotris woworae sp. nov., holotype MZB.25313 from Bali, Indonesia (86.4 mm SL); BIF2782
Distribution
Currently known from Bali. It was found in sympatry with E. fusca and E. melanosoma.
Ecology
The species is thought to be amphidromous. It lives in the lower part of the river, in lentic zones, where the velocity is weak and with mixed muddy-rocky bottoms with Oryzia sp (Teleostei, Adrianichthyidae) and near tide influence at one meter in elevation.
Eleotris sumatraensis sp. nov. Mennesson, Keith, Sukmono, Risdawati, Hubert
Figures 1, 2, 6; Tables 2–4
Material examined
Four males and one female collected from Sumatra with a size range of 45–68 mm SL.
Type material
Holotype: MZB.25315, male (62 mm SL), Sumatera Selatan, Benkulu, Tumbuan Sungai River, Sumatra, 22 Nov. 2015, coll. Hubert et al., BIF 4865.
Paratypes: MZB.25314, 2 males (45–47 mm SL), same data as holotype, BIF 4863 & 4864. MNHN 2019-0253, 1 male (68 mm SL), same data as holotype, BIF 4866. MNHN 2019-0254, 1 female (46 mm SL), West Sumatra, Padang, Air Turjun Lubuk Hitam, 1 May 2016, coll. Hubert et al., BIF 6068.
Other material (N = 48)
For the other species studied and compared in the 2.3.4 ‘melanosoma group’ pattern, see ‘Comparative material’.
Diagnosis
The species is distinguished by second, third and fourth suborbital free neuromast rows on cheek extending ventrally past horizontal row d (‘2.3.4’ ‘melanosoma group’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern), 16–17 pectoral fins rays, 31–33 scales in lateral series and 9–11 in zigzag series. A single mitochondrial lineage (BOLD: ADF2921).
Description
Scale and rays counts in Eleotris sumatraensis and related species are given in Tables 2 and 3, selected morpho-meristics in Table 4. Below, the holotype counts are given first, followed in brackets, if different, by the paratypes’ counts.
The body is elongate. The body depth at anus is 18% (15–20% SL) and the caudal peduncle depth is 13% (13–14% SL). Predorsal length 47% (45–49% SL) and preanal length 64% (65–70% SL). [End Page 485]
The head 33% (31–37% SL) is depressed and broad, the snout is pointed. Eyes high on head. The mouth is large, as jaw length is 11% (9–14% SL), and oblique, the posterior margin of upper jaw reaches the vertical through the first fourth of the eye. Upper and lower jaws with multiple rows of small teeth. Tubular anterior nares overhanging half upper lip, posterior nares open pits.
Dorsal fins VI-I,8; D1 separate from and smaller than D2; spines not elongate. Anal fin I,8 and directly opposite to second dorsal fin. Pelvic fins separate, I,5. Pectoral fins 16 (16–17). Caudal fin pointed with 15 branched rays.
Cycloid scales on top of head, nape, pectoral-fin base, pre-pelvic region, and abdomen. Operculum mostly naked, cheek naked. Ctenoid scales covering flanks. Scales in lateral series 47 (47–51); in predorsal series 32 (31–33), in transverse back series 14, in transverse forward series 19 (19–22) and in zigzag series 9 (9–11).
Gill opening is moderately broad, extending to below the preoperculum. Outer gill rakers on the first gill arch, they are thin, curved and tooth-shaped, except the two first on inner side, which are more rounded.
Cephalic lateralis: adults with 5 transverse suborbital free neuromast rows of which second, third and fourth on cheek extend ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed pattern’) (Figure 1D ).
Urogenital papilla in females rounded, elongated and tapered in males.
Eleotris sumatraensis sp. nov. (A) Holotype MZB.25315 from Sumatra, Indonesia (62 mm SL); BIF 4865. (B) Paratype MZB.25314 from Sumatra, Indonesia (57 mm SL); BIF 4864.
Colour in life (Figure 6A, 6B)
Male and female similar. Two different patterns in both sex. The first one with top of head, top of the eye and back beige to light brown. Lips greyish with several small brown blotches. Cheeks and operculum greyish with scattered small dark/pale spots. A large dark brown band from snout, through the eye and to the base of pectoral fin. Lateral part of body brown, with the central part of scales dark brown. Abdomen and gular region greyish. First dorsal fin with 5 greyish bands alternating [End Page 486] with 5 translucent bands; rays with yellow to orange spots alternating with black wavy spotted rows. Second dorsal, anal and pelvic fins with yellow to orange spots on rays alternating with black wavy spotted rows. Caudal fin dark brown with vertical rows of small beige dots. Pectoral fins translucent, with a vertical white band at the base and a white blotch at the upper part of this base. Pelvic with yellow to orange spots alternating with black wavy spotted rows. The second pattern with top of head, top of the eye and back yellowish. Lips whitish. Cheeks and operculum greyish to whitish with scattered small dark/pale spots. A large dark brown band from snout, through the eye and to the base of pectoral fin. Lateral part of body greyish to whitish, as abdomen and gular region. Pectoral fins yellowish and translucent. Pelvic whitish.
Colour in preservation
Head, preoperculum and body brown. Abdomen and gular region greyish. First dorsal fin with small dark spots alternating with small greyish ones. Anal and second dorsal fins with 5 brownish wavy spotted rows. Caudal, pectoral and pelvic fins greyish.
Etymology
The name for the new species is derived by combining Sumatra and the Latin suffix -ensis that in combination means ‘from or of Sumatra’.
Comparison
E. sumatraensis differs from the other species sequenced with a ‘2.3.4’ pattern and present in the area by displaying reciprocal monophyly from its closest relatives and high TPM1uf+G genetic distances to its relatives at COI gene (9.4% to 14.7% of divergence). Moreover, it differs from E. macrolepis in having 16–17 rays in pectoral fins versus 15 and from E. douniasi (this paper) and E. woworae (this paper) in having 16–17 rays in pectoral fins versus 19–20. It differs from E. melanosoma in having 31–33 versus 37–44 scales in predorsal series, and 9–11 scales in zigzag series versus 11–13.
Distribution
Currently known only from Sumatra. It was found in sympatry with E. fusca and E. melanosoma.
Ecology
The species is thought to be amphidromous. The specimens were found at 20 m high in elevation, in running freshwater, with mixed sand/gravel bottoms, boulders, biofilm and sediment covers, and with some macrophytes on edges. They are carnivorous (small fishes and shrimps).
Eleotris douniasi sp. nov. Keith, Mennesson, Dahruddin, Hubert
Figures 1, 2, 7; Tables 2–4
Material examined
Thirteen males and three females collected from Kalimantan with a size range of 42–95.5 mm SL.
Type material
Holotype: MZB.25317, male (70 mm SL), Kalimantan, Kalimantan Utara, Setulang, Hilir, Indonesia, 18 Nov. 2018, coll. Hubert et al., BIF 9880.
Paratypes: MZB.25318, 3 males and 2 females (46–59 mm SL), same data as holotype, BIF 9879 & 9881 to 9884.-MNHN 2019-0255, 1 male and 1 female (69–95.5 mm SL), same data as holotype, BIF 9877 & BIF 9878.-MZB.25316, 6 males (42–77 mm SL), Kalimantan, Kalimantan Utara, Setulang, Setulang, Indonesia, 18 Nov. 2018, BIF 9776, 9777 & 9780 to 9783.-MNHN 2019-0256, 2 males (63–66.5 mm SL) Kalimantan, Kalimantan Utara, Setulang, Setulang, Indonesia, 18 Nov. 2018, BIF 9778 & BIF 9779.
Other material (N = 37)
For the other species studied and compared in the 2.3.4 ‘melanosoma group’ pattern, see ‘Comparative material’.
Diagnosis
The species is distinguished by second, third and fourth suborbital free neuromast rows on [End Page 487] cheek extending ventrally past horizontal row d (‘2.3.4’ ‘melanosoma group’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed’ pattern), 19–20 pectoral fins rays, 41–44 scales in lateral series and 31–37 in predorsal series. A single mitochondrial lineage (BOLD: AEA5081).
Description
Scale and rays counts in Eleotris douniasi and related species are given in Tables 2 and 3, selected morpho-meristics in Table 4. Below, the holotype counts are given first, followed in brackets, if different, by the paratypes’ counts. The body is elongate.
The body depth at anus is 19% (16–20% SL) and the caudal peduncle depth is 14% (12–15% SL). Predorsal length 45% (43–47% SL) and preanal length 70% (64–70% SL).
The head 32% (31–34% SL) is depressed and broad, the snout is pointed. Eyes high on head. The mouth is large, as the jaw length is 10% (9–12% SL), and oblique, the posterior margin of upper jaw reaches the vertical through the first fourth of the eye. Upper and lower jaws with multiple rows of small teeth; a few caniniform teeth in posterior position. Tubular anterior nares overhanging half upper lip, posterior nares open pits.
Dorsal fins VI-I,8; D1 separate from and smaller than D2; spines not elongate. Anal fin I,9 and directly opposite to second dorsal fin. Pelvic fins separate, I,5. Pectoral fins 19 (19–20). Caudal fin pointed with 15-branched rays.
Cycloid scales on top of head, nape, pectoral-fin base, pre-pelvic region and abdomen. Operculum mostly naked, cheek naked. Ctenoid scales covering flanks. Scales in lateral series 43 (41–44), in predorsal series 34 (31–37), in transverse back series 14 (14–16), in transverse forward series 22 (19–24) and in zigzag series 11 (11–12).
Gill opening is moderately broad, extending to below the preoperculum. Outer gill rakers on the first gill arch 9–10, they are thin, curved and tooth-shaped, except the two first on inner side which are more rounded.
Cephalic lateralis: adults with 5 transverse suborbital free neuromast rows of which second, third and fourth on cheek extend ventrally past horizontal row d (‘2.3.4’ pattern); row os connected with row oi at ventro-posterior margin of operculum (‘closed pattern’) (Figure 1D ).
Urogenital papilla in females rounded, elongated and tapered in males.
Eleotris douniasi sp. nov., holotype MZB.25317 from Kalimantan, Indonesia (70 mm SL); BIF 9880
Colour in life (Figure 7)
Male and female similar. Entire body mainly dark brown. Cheeks and operculum slight brown with numerous yellowish dots from the snout to the operculum. Lips brownish banded with several small yellowish blotches. Lateral part of body dark brown, with several yellowish scales, forming sometimes irregular lines. Abdomen and gular region greyish with scattered small dark/pale spots. First dorsal fin with 2 black bands alternating with 2–3 yellowish bands; rays with yellow to orange [End Page 488] spots alternating with black wavy spotted rows. Second dorsal, anal and pelvic fins with yellow spots on rays alternating with black wavy spotted rows. Caudal fin dark brown with vertical rows of small yellowish dots. Pectoral fins with yellowish spots alternating with black wavy spotted rows along rays; a reticulated yellowish spot at the base. Pelvic greyish with black rays.
Colour in preservation
Entire body mainly dark brown. Lips brownish banded with several small greyish blotches. Abdomen and gular region greyish with scattered small dark/pale spots. First dorsal fin with 2 black bands alternating with 2–3 greyish bands. Second dorsal, anal and pelvic fins with greyish spots on rays alternating with black wavy spotted rows. Caudal fin dark brown with vertical rows of small greyish dots. Pectoral fins with greyish. Pelvic greyish with black rays.
Etymology
The new species is named douniasi in dedication to Edmond Dounias from IRD, Indonesia, for his kindness and for facilitating field missions in Indonesia for several years.
Comparison
E. douniasi differs from the other species sequenced with a ‘2.3.4’ pattern and present in the area by displaying reciprocal monophyly from its closest relatives and high TPM1uf+G genetic distances to its relatives at COI gene (10.9% to 15.5% of divergence). Moreover, it differs from all the other species of the area except E. woworae (this paper), in having 19–20 rays in pectoral fins versus 15–17. It differs from E. woworae in having fewer scales in lateral series (41–44 versus 55).
Distribution
Currently known only from Kalimantan.
Ecology
This species lives in the lower part of the rivers, in fresh muddy to clear water. It is carnivorous (small fishes, molluscs and shrimps).
Eleotris macrocephala, holotype RMNH 25935, from Buru, Indonesia (76.5 mm SL)
1-a: Cephalic neuromast pattern ‘closed; 2.4.6’ (Figure 1C ): 2
1-b: Cephalic neuromast pattern ‘open; 2.4’ (Figure 1B ): E. acanthopomus (Figure 10)
1-c: Cephalic neuromast pattern ‘closed; 2.3.4’ (Figure 1D ): 3
1-d: Cephalic neuromast pattern ‘closed; 2.4.5.6’ (Figure 1E ): E. eigenmanni (Figure 9)
2-a: Lateral scales <43. Transverse scales in backward series 12: E. macrocephala (Figure 8)
2-b: Lateral scales >50. Transverse scales in backward series 15–21: E. fusca (Figure 11)
Key of Eleotris species from Indonesia
[End Page 489]
Eleotris eigenmanni, syntype RMNH 10708, from Sunda Islands, Indonesia (78.4 mm SL)
Eleotris acanthopomus, from Lombok, Indonesia (40 mm SL); BIF 3999
Eleotris fusca, from Lombok, Indonesia (73 mm SL); BIF 3989
3-a: Pectoral rays 15: E. macrolepis (Figure 4)
3-b: Pectoral rays 16–17: 4
3- c: Pectoral rays 19 or 20: 5
4- a: Predorsal scales 37–44, Zigzag scales 11–13: E. melanosoma (Figure 3) [End Page 490]
4- b: Predorsal scales 26–33, Zigzag scales 9–11: E. sumatraensis (Figure 6)
5-a: Lateral scales 55, Zigzag scales 13: E. woworae (Figure 5)
5-b: Lateral scales 41–44, Zigzag scales 11–12: E. douniasi (Figure 7)
Comparative material
Neuromast pattern ‘2’
Types examined:
Eleotris oxycephala Temminck & Schlegel, 1845: 1 possible type from Japan (BMNH 2015.4.8.1).
Eleotris balia Jordan & Seale, 1905: holotype from China (USNM 52082).
Pattern ‘2.4’
Types examined:
Eleotris sandwicensis Vaillant & Sauvage, 1875: 4 syntypes from Hawaiian Islands (MNHN A-2624, 8045, 8915, 9011).
Eleotris aquadulcis Allen & Coates, 1990: 3 paratypes from Papua New Guinea (WAM P.29608-002).
Eleotris acanthopomus Bleeker, 1853: holotype from Sumatra, Indonesia (RMNH 25934).
Other specimen, non-type, of E. acanthopomus: Pacific Ocean, Cook Islands: MNHN 2016–0026, Rarotonga, July 2010, tag 16005, Keith et al. coll.- French Polynesia: MNHN 2020-0218, 6 specimens, Society Islands, Moorea, June 2007, tag 16102, Sasal et al. coll.- MNHN 2020-0219, 3 specimens, Society Islands, Moorea, June 2007, tag 12428, Sasal et al. coll.- Vanuatu : MNHN 2020-0215, 8 specimens, Gaua, Kaska riv., 05 Nov. 2014, tag 13548, Keith et al. coll.- MNHN 2016-0027, Gaua, Kaska riv., 05 Nov. 2014, tag 13546, Keith et al. coll.- Micronesia: MNHN 2020-0214, 2 specimens, Pohnpei, riv. Petroglyphe, 14 Feb. 2013, tag 12476, Keith et al. coll.- Solomon Islands: MNHN 2016-0028, 3 specimens, Koloban- gara, Vanga riv., 18 Nov. 2015, tag L-252, Keith et al. coll.- Papua New Guinea: MNHN 2020-0048, New-Britain, Oso resurgence, 27 Oct. 18, tag 17690, Keith et al. coll.-Indonesia: MZB, uncatalogued, Sulawesi , Sulawesi Tengah, Ampana, Tongku, 15 June 2019, Hubert et al. coll., BIF10334.- MZB, uncatalogued, 2 specimens, Java, West Java, Kab Sukabumi, Ci Tepus, 22 Nov. 2012, Hubert et al. coll., BIF0305, BIF0306.- MZB, uncatalogued, 5 specimens, Bali, West Bali, Kab Jembrana, Nbang, 15 Apr. 2014, Hubert et al. coll., BIF2308, BIF2308, BIF2312, BIF2313, BIF2314.- MZB, uncatalogued, Lombok, Lombok utara, Sidutan, 28 March 2015, Hubert et al. coll., BIF3806.- MZB, uncatalogued, 2 specimens, Lombok, Lombok selatan, Kali Pudak, 1 Apr. 2015, Hubert et al. coll., BIF3999, BIF4000.- MZB, uncatalogued, 3 specimens, Lombok, Lombok utara, Kali Sidutan, 2 Apr. 2015, Hubert et al. coll., BIF4032, BIF4033 & BIF4034.
Indian Ocean, Comoros Islands: MNHN 2006-0617, 3 specimens, Moheli, Mdjawaché, 31 Oct. 2005, Keith et al. coll.- MNHN 2020-0029, Mayotte, Kwale, Comoros Islands, 22 Apr. 2009, Feutry coll.; tag 12426.- MNHN 2020-0030, Comoros Islands, Mayotte, Apr. 2009, Feutry coll.; tag 12417.- MNHN 2020-0031, Mayotte, Our-oveni, Comoros Islands, 06 Sept. 2007, Marquet coll.; tag 13951 MNHN 2020-0032, same data as 2020-0031, 06 Sept. 2007, Marquet coll.; tag 13952.- MNHN 2020-0033, same data as 2020-0031; tag 13953.- MNHN 2020-0034, same data as 2020-0031; tag 13954.- MNHN 2020-0041, Mayotte, Ouroveni, Comoros Islands, 06 Sept. 2007, Marquet coll.; tag 11817.- MNHN 2020-0035, Mayotte, Coconi, Comoros Islands, 06 Aug. 2019, Valade et al. coll.; tag 14519.- MNHN 2020-0036, same data as 2020-0035, tag 14520.- MNHN 2020-0037, same data as 2020-0035, tag 14521.- MNHN 2020-0038, Comoros Islands, Mayotte, Ouroveni, 06 Aug. 2019, Valade et al. coll.; tag 14562.- MNHN 2020-0039, Comoros Islands, Mayotte, Longoni, 07 Aug. 2019, Valade et al. coll.; tag 14563.- MNHN 2020-0040, same data as 2020-0039, tag 14564.- Seychelles Islands: MNHN 2007-0185, 2 specimens, Mahé, Seychelles Islands, 24 Nov. 2004, Accouche et al. coll.- MNHN 2007-0199, Mahé, Anse aux poules bleues riv., Seychelles Islands, 14 Oct. 2003, Keith et al. [End Page 491] coll.- MNHN 2007-0205, Praslin, Fond B’Offay riv., Seychelles Islands, 10 Oct. 03, Keith et al. coll.; tag 14509.- MNHN 2020- 0042, Praslin, Nouvelle découverte, Sey- chelles Islands, 09 Oct. 2003, Keith et al. coll.; tag 13749.-MNHN 2007-0200, 1 (tag C) of 3, Mahé, Seychelles Islands, 22 Nov. 2004, Accouche et al. coll.
Pattern ‘2.3.4’
Types examined:
Eleotris macrolepis (Bleeker, 1875): 2 syntypes from Ambon, Indonesia (RMNH 4759).
Eleotris melanosoma Bleeker, 1853: 3 syntypes from Wahai, Ceram, Indonesia (in RMNH 4815). The following synonyms were studied: Culius insulindicus Bleeker, 1875: 2 syntypes from Sumatra, Indonesia (RMNH 4804).- Eleotris pseudacanthopomus Bleeker, 1853: holotype from Western Sumatra, Indonesia (SMNS 10595).- Eleotris melanura Bleeker, 1849: holotype from southern Java, Indonesia (in RMNH 5182).
Other specimens, non-type, of E. melanosoma: Solomon Islands: MNHN 2016-0030, Kolobangara, Vage river, 10 Nov. 15, Keith et al., tag 12397.- MNHN 2016-0031, Solomon Islands, Kolobangara, Vanga riv., 18 Nov. 15, Keith et al., tag 12487.- MNHN 2016-0032, Solomon Islands, Kolobangara, Zamba riv., 10 Nov. 15, Keith et al., tag L-229. MNHN uncatalogued, Solomon Islands, Choiseul, Tutuku, 19 Oct.19, Keith et al. coll.; tag 17597. Indonesia : MZB.25307, Java, Ci Tepus, 22 Nov. 2012, Hubert et al., BIF 0307 & 0309.- MZB.25308, Java, Ci Mandiri, 24 Nov. 2012, Hubert et al., BIF 0379.- MZB.25309, Java, Kab Pandeglang, 09 Dec. 2013, Hubert et al., BIF 1567 & 1568. - MZB uncatalogued, Java, BantenKab Pandeglang, Cibeber, 12 Jul. 2013, Hubert et al., BIF 1471.- MZB.25310, Java, Kab Lumajang, 13 Apr. 2014, Hubert et al., BIF 2168 & 2169.- MZB.25319, Bali, Kab Kelungkung, 22 Apr. 2014, Hubert et al., BIF 2787.- MZB uncatalogued, Bali, Kab Kelungkung, 22 Apr. 2014, Hubert et al., BIF 2784.- MZB.25311, Java, Kab Lumajang Mujur, 13 Apr. 2014, Hubert et al., BIF 2177 & 2178.- MZB.25312, Sumatra, Air Turjun Lubuk Hitam, 01 May 2016, Hubert et al., BIF 6067.- MNHN uncatalogued, Papua New Guinea : New Britain, Swamp river, Rangihi, 24 Nov. 18, Keith et al. coll., tag 17750.- MNHN uncatalogued, New Britain, Swamp river, Rangihi, 24 Nov. 18, Keith et al. coll.tag 17751.- MNHN uncatalogued, Papua New Guinea, New Britain, Swamp river, Rangihi, 25 Nov. 18, Keith et al. coll., tag 17677.- MNHN uncatalogued, Papua New Guinea, New Britain, Swamp river, Rangihi, 25 Nov. 18, Keith et al. coll., tag 17680. MNHN uncatalogued, New Caledonia: Grande Terre, Poro-Waneubwayo, 01 June 2016, Charpin coll., tag 18288.
Pattern ‘2.4.6’
Types examined:
Eleotris macrocephala (Bleeker, 1857): holotype from Buru, Indonesia RMNH 25935.
Eleotris bosetoi Mennesson, Keith, Ebner and Gerbeaux, 2016: holotype (MNHN-IC-2015-0382) and paratypes (MNHN-IC-2015-0380, MNHN-IC-2015-0379, MNHN-IC-2016-0001) from Solomon Islands.
Eleotris fusca (Bloch & Schneider, 1801): no type known. The following synonyms were studied: Eleotris nigra Quoy and Gaimard, 1824: syntype from Waigeo, Indonesia (MNHN-IC-A-1578). Eleotris vitianus Sauvage, 1880: 2 syntypes from Fiji Islands (MNHN-IC-A-1420). Eleotris fornasini Bianconi, 1857: holotype from Mozambique, Africa (BMNH 1852.9.13.179). Eleotris andamensis Herre, 1939: 2 paratypes from Andaman Islands (CAS-SU 37152). Eleotris brachyurus Bleeker 1849: syntype from Patjitan, southern Java, Indonesia (RMNH 5182).
Other specimens, non-type, of E. fusca: Samoa: MNHN 2015-0364, 2 specimens, Samoa, Upolu, 25 July 2008, Keith et al. coll., tags 16023 & 16024.-MNHN 2015-0376, Samoa, Upolu, Palilua riv., 25 July 2008, Keith et al. coll., tag 16020.-Indonesia: MNHN 2015-0365, 3 specimens, Bali, Tukad Unda, 22 Apr. 2014, Keith et al. coll., tags 12443, 12446 & 12447.-MNHN 2015-0367, Kumafa, Papua, 15 Oct. 2010, Keith et al. [End Page 492] coll., tag 16015.-MNHN 2015-0368, Tireloach, Palau, 28 Feb. 2011, Keith et al. coll., tag 16017.-MNHN 2015-0369, Pohnpei, 14 March 2012, Keith et al. coll., tag 16019.- Solomon: MNHN 2015-0370, Lokapava, Choiseul, 21 Oct. 2014, Keith et al. coll., tag 13531.- Vanuatu, MNHN 2015-0371, Maewo, 12 Nov. 2007, Keith et al. coll., tag 16124.- MNHN 2015-0378, Vanuatu, Epi, Buavinai, 27 Nov. 2014, Keith et al. coll., tag 13526.- French Polynesia: MNHN 2015- 0372, Moorea, June 2007, Sasal et al. coll., tag 16097.- MNHN 2015-0373, Rurutu, June 2001, Keith et al. coll., tag 16094.- MNHN 2015-0374, Tubuai, July 2007, Keith et al. coll., tag 16086. - MNHN 2015-0366, Ua Uka, Marquesas, 24 Feb. 2009, Pascal et al. coll., tag 16087. - Philippines: MNHN 2015- 0375, Alegre, 5 Feb. 2014, tag 12450.-MNHN 2015-0377, Papua, 26 Oct. 2008, Keith et al. coll. tag 16018.- MNHN 2015- 0383, New Caledonia, Wan Pwé On, 02 March 2013, Taillebois et al. coll., tag L-207. Reunion Island: MNHN 2020-0076, 2 specimens, Mascarene Islands, Saint Jean, 24 March 2007, Arda coll., tags 11823 & 11825.
Pattern ‘2.4.5.6’
Types examined:
Eleotris eigenmanni Popta, 1921: 7 syntypes from Sunda Islands, Indonesia (RMNH 10708 (1); SMF 6594 (1) and SMF 6595-99 (5)).
Museum für Naturkunde, Leibniz-Institut für Evolutions und Biodiversitätsforschung an der Humboldt-Universität zu Berlin, Invalidenstrasse 43, Berlin 10115, Germany.
acknowledgments
We wish to thank Bambang Dwisusilo, Sumanta, Daisy Wowor and Ujang Nurhaman for their help during the field sampling. Part of the present study was funded by the MNHN (UMR 7208 BOREA), the ‘Institut de Recherche pour le Développement’ (UMR ISEM), the Indonesian Institute of Sciences (LIPI), the French Ichthyological Society (SFI) and the Fondation de France. This study has been approved by the Indonesian Ministry of Research & Technology (MEN-RISTEK) and field sampling has been conducted according to the research permits for Philippe Keith (75/SIP/FRP/E5/Dit.KI/III/2017), and the research permit for Nicolas Hubert (50/EXT/SIP/FRP/E5/Dit.KI/IX/2016). We wish to thank MENRISTEK staffs as well as Mohammad Irham, Ruliyana Susanti, Gina Naandriana, Rosichon Ubaidillah, Hari Sutrisno and Witjaksono (Research Center for Biology-LIPI) for the research permits and supporting letters. For the loan of specimens, we thank: R. Hadiaty, D. Wowor (MZB), D. Catania (CAS), R. de Ruiter & E. Dondorp (RMNH), S. Dorow, H. Zetzsche, T.J. Alpermann and F. Krupp (SMF), S. Morrison and G.R. Allen (WAM), S. Merker (SMNS), I. Eidus (ZMH), T. Heath and J. Maclaine (BMNH), L. Parenti, J. Williams, L. Palmer and S. Raredon (USNM), M. Hammer and G. Dally (NTM), P. Pruvost, R. Causse, Z. Gabsi, J. Pfliger (MNHN).
This is publication ISEM 2020_223 SUD.