First Records and Descriptions of Early Life Stages of Cephalopods from Rapa Nui (Easter Island) and the Nearby Apolo Seamount1
New records of early life stages of cephalopods are presented based on planktonic collections carried out around Easter Island (Rapa Nui; 27°7′S, 109°21′W) and at the nearby Apolo Seamount (located at ~7 nautical miles southwest from Easter Island) during March and September 2015 and March 2016. A total of 13 individuals were collected, comprising four families (Octopodidae, Ommastrephidae, Chtenopterygidae, and Enoploteuthidae) and five potential genera/types (Octopus sp., Chtenopteryx sp., rhynchoteuthion paralarvae, and two undetermined Enoploteuthid paralarvae). Cephalopod mantle lengths (ML) ranged from 0.8 to 4.5 mm, with 65% of them (mainly Octopodidae) corresponding to newly hatched paralarvae of ~1 mm ML, and 35% to rhynchoteuthion and early stages of oceanic squids of around 1.5–4.5 mm ML. These results provide the first records on composition and presence of early stages of cephalopods around a remote Chilean Pacific Island, while also providing a morphological and molecular basis to validate the identity of Octopus rapanui (but not Callistoctopus, as currently recorded), Ommastrephes bartramii and Chtenopteryx sp. around Rapa Nui waters. Despite adult Octopodidae and Ommastrephidae having been previously recorded at these latitudes, the current findings provide evidence to suggest that the northwest side of Easter Island, and one of the nearby seamounts, may provide a suitable spawning ground for benthic and pelagic species of cephalopods inhabiting these areas. For Chtenopterygidae and Enoploteuthidae, this is the first record for the Rapa Nui ecoregion.
Chtenopterygidae, Enoploteuthidae, octopus, Octopus rapanui, Ommastrephidae, paralarvae
The first zoological studies conducted in the Southeastern Pacific (SEP) oceanic environments began in 1950 s, with detailed investigations on the fauna associated to submarine ridges being carried out between 1973 and 1987 during several Russian cruises (Parin et al. 1997). At that time, the seamounts around the Salas y Gómez and Nazca ridges were also explored, highlighting the close relationship of benthic and benthopelagic [End Page 163] invertebrates and fishes with the Indo-west Pacific fauna and the very high degree of endemism at the species levels (for details see Parin et al. 1997). Since then, several ecological studies have emphasized the endemism patterns recorded in these and other oceanic environments around the SEP, including the Easter and Salas y Gómez Islands (Friedlander et al. 2013, Fernández et al. 2014, Hernández et al. 2015), and the Juan Fernández Archipelago and Desventuradas Islands (Pérez-Matus et al. 2014, Friedlander et al. 2016).
On the Nazca Plate in the southeast subtropical Pacific, Easter, and Salas y Gómez Islands maintain a high diversity of macroalgae, invertebrates and fishes (964 species), with some groups showing high levels of endemism (e.g. mollusks and poriferans with 33% and 34%, respectively) (Rehder 1980, Fernández et al. 2014). Commonly, invertebrate and vertebrate eggs and larvae of oceanic and insular species are concentrated around oceanic islands (Boehlert et al. 1992, Boehlert and Mundy 1993, Parin et al. 1997, Castro and Landaeta 2002). Around Easter and Salas y Gómez Islands, fish larvae are very abundant and diverse (> 70 species) (Castro and Landaeta 2002, Landaeta et al. 2002, 2003, 2005, Acuña and Cabrera 2007); however, invertebrates are less studied and efforts have been concentrated mainly on crustaceans (< 30 species) (Robledo and Mujica 1999, Rivera and Mujica 2004, Mujica 2006, Meerhoff et al. 2017, Díaz-Cabrera et al. 2017). In this context, further field information on highly diverse and/or endemic invertebrate species (e.g. mollusks) is still needed, particularly on species distributions and critical life-history traits, including reproductive biology, size at maturity, egg size, and larval/juvenile stages.
For example, for mollusks in general and for cephalopods in particular (e.g. octopuses and squids), they correspond to less diverse groups inhabiting Rapa Nui (Rehder 1980, Fernández et al. 2014); nonetheless, it is plausible that their early life stages (as in fishes) are present in offshore waters around the island, as has been described in Hawaii and off Brazil (Young and Hirota 1990, Bower et al. 1999, Haimovici et al. 2002). Around the Hawaiian Islands, Bower et al. (1999) collected 57 species of cephalopods belonging to two paralarval assemblages, island-associated and oceanic, suggesting that both groups may select these areas as spawning grounds. At Easter Island, few cephalopod species have been recorded to date, including one endemic octopus Callistoctopus rapanui (Voss 1979) and three squids: Ommastrephes bartrami (Lesuer, 1821), Eucleoteuthis luminosa (Sasaki, 1915), and Nototodarus hawaiiensis (Berry, 1912) (Voss 1979, Prado 1983, Okutani and Kuroiwa 1985, Nesis 1993). Additionally, Parin et al. (1997) reported from the Salas y Gómez ridge (~400 km east from Rapa Nui) two benthopelagic octopods, Scaeurgus unicirrhus (Delle Chiaje [in Férussac & d’Orbigny], 1841) and Pteroctopus hoylei (Berry, 1909), and four potential species of sepiolids, including Heteroteuthis dispar (Rüppell, 1845), Iridoteuthis maoria Dell, 1959, Sepioloidea pacifica (Kirk, 1882) and Stoloteuthis leucoptera (Verrill, 1878).
Considering that most holopelagic and merobenthic cephalopod species possess small planktonic paralarvae that can be transported by currents after hatching (a powerful dispersal mechanism; Villanueva et al. 2016), it is plausible to expect that early stages of at least six species of cephalopods (except holobenthic species such as some octopuses) could be found around Easter Island. However, as most paralarval and juvenile stages are difficult to capture (Sweeney et al. 1992, Rodhouse et al. 1992), it is not surprising that they have been underrepresented in planktonic and/or benthic surveys.
Adding to the available knowledge of cephalopod life-history stages in Southeast Pacific waters, the current study aims to provide new information on morphologic and molecular characterization, size structure, and geographic distribution of early life stages of cephalopods collected around the remote Rapa Nui (Easter Island) and the nearby Apolo Seamount.
materials and methods
Field Collections and Morphological Characterization
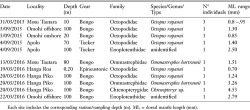
Early life history stages of cephalopods were collected from zooplankton samplings conducted in the coastal zone of Rapa Nui and [End Page 164] the nearby Apolo Seamount (located ~13 km southwest from Easter Island) on board of the vessel “Toke Rau” (Chilean Navy; March 2015) and also from fishing boats (September 2015, March 2016). Sampling was conducted in eleven stations during three oceanographic campaigns carried out in March and September 2015, and March 2016 (Table 1; Figure 1). In each station, samples were obtained with oblique zooplankton tows conducted by deploying a Bongo net (300 mm mesh; 0.4 m diameter) or a Tucker trawl (300 mm mesh; 0.25m2) down to 200m or to a safe depth above the bottom around shallower stations (Meerhoff et al. 2017). Day and night epineustonic samplings were also carried out over the course of three consecutive nights during March 2015 and subsequently only during daytime in September 2015 and March 2016 (Table 1). All oblique and epineustonic tows were performed at a constant velocity of 2 knots, and the volume filtered was estimated using a mechanical flowmeter (Sea Gear), ranging from 300 to 500 m3 in epineustonic samplings and from 300 to 900 m3 with Bongo nets (Meerhoff et al. 2017). Zooplankton samples were preserved in ethanol (96%) until further examination. Once in the laboratory and during the sorting process, all cephalopod paralarvae were extracted from the entire sample and each individual was morphologically identified to the lowest taxonomic level by comparison with descriptions available in the literature using standardized morphological characters, including dorsal mantle length (ML, mm), number and distribution of suckers (S), shape of tentacular club (TC) and chromatophore patterns (Young and Hirota 1990, Sweeney et al. 1992, Vecchione et al. 2001, Haimovici et al. 2002, González et al. 2008, Uriarte et al. 2010, Carrasco 2014, Zaragoza et al. 2015, Fernández-Álvarez et al. 2016). All specimens were measured following Roper and Voss (1983), and using a dissecting microscope equipped with an ocular micrometer at 20X or 50X magnification, depending on the species and structures evaluated. Specimens obtained in Rapa Nui were added to the collection maintained at Sala de Colecciones Biológicas, Universidad Católica del Norte (SCBUCN), and further compared with individuals stored there using their corresponding catalogue identification number.
Early life history stages of cephalopods collected around Rapa Nui (Easter Island; 27° S; 109° W) and at the nearby Apolo Seamount (~13 km southwest from Easter Island)
Molecular Analysis
DNA was successfully extracted from three complete paralarvae (i.e. Octopodidae, Ommastrephidae and Chtenopterygidae) and [End Page 165] from one juvenile Octopus rapanui previously obtained from Tahai, north of Hanga Roa, in 2014 (8.6 mm ML; SCBUCN#6899), using the salting-out technique (Aljanabi and Martinez 1997). The primers used were those proposed by Folmer et al. (1994) to amplify the mitochondrial gene Cytochrome Oxidase I (COI). PCR amplifications for each paralarva used the following protocol: 0.3 μl TaqDNA polymerase (1.5 U), 2.5 μl 10X(50 mM KCl, 10 mM Tris-HCl, pH 8.0) commercial buffer, 2 μl dNTPs (10 μM), 1.0 μl 50 mM MgCl2 and 0.5 μl (10pg/μl) of each primer. Optimum amplification conditions were used in a thermocycler: initial denaturation at 94°C for 3 minutes (min), followed by 35 amplification cycles of denaturation at 94°C for 40 seconds (s), annealing at 50°C (40 s), and 72°C (60 s), with a final extension at 72°C (7 min). The amplicons were purified and sequenced by Macrogen Inc. (South Korea). Finally, the sequences were edited and aligned by eye using the program ProSeq version 2.9 (Filatov 2002). To genetically identify the paralarvae, we performed a nucleotide BLASTIN search in NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch) and used the identification engine in the BOLD database (http://barcodinglife.org/index.php/IDS_OpenIdEngine). Sequences generated in this study are available in GenBank under the codes (ORP1, ORJ1, OBP1, and CHP1).
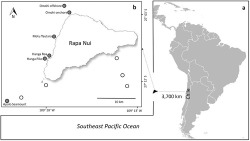
(A) Representation of the Southeast Pacific Ocean with an approximate location of Rapa Nui (Easter Island; 27° S, 109° W), and (B) sampling locations around Rapa Nui, with negative (white circles) and positive (gray circles) stations where cephalopod paralarvae were collected.
results
In the present study, cephalopod paralarvae were found in six of the 11 zooplankton stations sampled, corresponding to relatively shallow stations not exceeding 100 m depth and located close to land masses, including mainland Rapa Nui and its associated islets (motus), and the nearby Apolo seamount. A total of 13 specimens were caught, including the families Octopodidae d’Orbigny, 1840, Ommastrephidae Steenstrup, 1857, Chtenopterygidae Grimpe, 1922, and Enoploteuthidae Pfeffer, 1900. Systematics, distribution and species-specific morphological and genetic traits are provided in Table 1, Figures 1 and 2, as well as in the following descriptions. [End Page 166]
Family Octopodidae
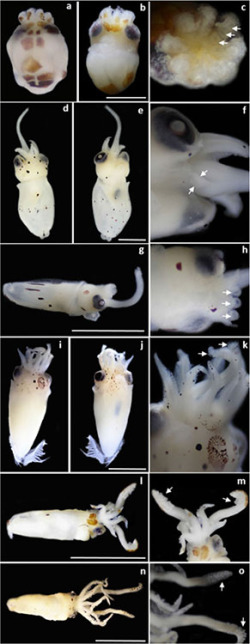
These individuals were the most abundant, with eight paralarvae ranging from 0.7 to 1.4 mm ML, being collected in most positive stations at the west side of the island, from Omohi offshore to the Apolo seamount (Table 1; Figure 1). All octopod paralarvae presented the same morphological traits (i.e. number of suckers, chromatophore patterns), suggesting they corresponded to the same species (Figures 2A, B). They had a muscular body covered by kölliker’s organs on arms and mantle, especially the smaller sizes. The mantle, short and rounded, had a mean length (mean ML± SD) of 1.02 ± 0.26 mm. Head width represented 64% of the ML. Eyes were relatively large, representing around 22% of ML. The funnel was small and did not exceed the posterior margin of eyes (24% of ML). Arms were shorter than the mantle, and in most cases were retracted due the preservative (Figures 2A, B). Each arm presented a uniserial line of three suckers of similar size (i.e. 9% of ML; Figure 2C). Arrangements of brown/orange chromatophores covered the dorsal and ventral surfaces of their bodies, varying in size and shape depending on its expansion. Each arm presented one basal chromatophore in the aboral surface. The dorsal surface of the head presented around — six to eight chromatophores commonly distributed in a pattern 2+2+4 counted from the base of the arm crown toward the dorsal mantle margin (i.e. anterior to posterior). Additionally, each eye presented one extrategumental chromatophore covering its dorsal surface (i.e. supraocular). The ventral surface of the head seemed to have two extrategumental chromatophores located one on each side above to the funnel. Funnel chromatophores were not distinguishable. The dorsal surface of the mantle lacked chromatophores, whereas the ventral surface presented two, usually located toward the posterior end (i.e. cup chromatophores). Over the perivisceral epithelium, a clear pattern of —five to six large tegumental chromatophores covered the dorsal surface of the visceral mass (see Figure 2A). Sequencing results of the mitochondrial gene COI for one of these paralarvae (GenBank [End Page 167] accession number: MH347314) and the field-collected juvenile (SCBUCN #6899; GenBank accession number: MH347313) evidenced a 94% (GenBank accession number: AB430544.1) and 94.3% (BOLD database) similitude with the Indo-West Pacific Octopus parvus (Sasaki, 1917). In the other hand, our COI sequences showed a low similitude (15%–16%; GenBank Blast) with Callistoctopus spp. These comparisons suggest that both stages (paralarvae and juvenile) corresponded to the same species within the Octopus group and not to the Callistoctopus group.
Early life-history stages of cephalopods collected around Rapa Nui (Easter Island) and the Apolo Seamount during 2015 and 2016. From top to bottom: Octopodidae, Ommastrephidae, Chtenopterygidae, and Enoploteuthidae. (A, B, C) Octopus rapanui, where (A) and (B) dorsal and ventral views, scale bar: 0.5 mm, (C) close-up of an oral view, where arrows indicate the three suckers per arm. (D–H) Ommastrephes bartramii, where (D) and (E) dorsal and ventral views of the larger specimen, (F) closeup of a ventral view, where arrows indicate undeveloped arms IV, (G) dorso-lateral right view of the smaller specimen, (H) closeup of a dorsal view, where arrows indicate the single sucker per arm, scale bars in D, E, and G: 1.5 mm. (I, J, K) Chtenopteryx sp., where (I) and (J) dorsal and ventral views, scale bar: 1.5 mm, (K) close-up of a dorsal view, where arrows indicate suckers in tentacles. (L–O) Enoploteuthidae, where (L) dorsal view of the smaller specimen, (M) close-up of a dorsal view, with arrows indicating suckers in tentacles, (N) dorsal view of the larger specimen, (O) closeup of a ventral view, with arrows indicating suckers in tentacles, scale bars in l and n: 2.5 mm.
Family Ommastrephidae
Two rhynchoteuthions were collected (Table 1), corresponding to the characteristic paralarvae of the family Ommastrephidae. These paralarvae were easily distinguished from other families by their fused tentacles, forming a proboscis (see Figures 2D–H).
The larger rhynchoteuthion had a rounded mantle of 3.26 mm (Figs. 2D–F; GenBank accession number: MH347315), with sequencing results of the mitochondrial gene COI evidencing a 99% (GenBank accession number: HQ829184.1) and a 100% (BOLD database) similitude with Ommastrephes bartramii. The head width represented 57% of the ML and the eyes around 16% ML. The funnel had a medium size, reaching the posterior margin of the eyes and representing 39% of the ML (Fig. 2D,E). The long proboscis reached on average ~86% of the ML, and seemed to have only small suckers on the tip (some possibly were detached due preservation). Arms presented different numbers of pedunculated suckers distributed in a biserial row, ranging from six in arm I, to 9–10 in arms II and III. Arm IV was present but not well developed (Fig. 2F). Small posterior and rounded fins (10% ML) were also observed. The chromatophore patterns were characterized by well-defined brown dots over the dorsal and ventral surfaces of the body. Arms lacked pigmentation, although two small chromatophores were observed ventrally in arm II. The dorsal head presented ten chromatophores distributed in a pattern 1+2+4+3, one between the arms I, two between the eyes, one pair posterior to each eye and three on the posterior head. Additionally, each eye presented one supra-ocular chromatophore. Ventral surface of head with four chromatophores in the posterior margin, two on each side of the funnel. A single chromatophore was present on the ventral surface of each eye. No funnel chromatophores were observed. The dorsal mantle showed six brown chromatophores, one anterior and five in a central row perpendicular to the mantle axis. The ventral mantle had a denser arrangement of around 22 chromatophores (Figures 2D,E). The small rhyncoteuthion collected presented a tubular mantle of 1.51 mm and similar morphological traits when compared with the above specimen (e.g. chromatophores patterns and pedunculated suckers) (see Figures 2G,H). Head width represented 48%, eyes 16%, and funnel 36% of the ML, respectively. The long proboscis (50%–75% ML) had two enlarged lateral suckers on the tip, up to twofold larger than the remaining six suckers. Each arm had one pedunculated sucker (Figure 2H). Brown/orange chromatophores spotted the dorsal and ventral surfaces of the paralarvae. Arms lacked pigmentation. The dorsal surface of the head presented three chromatophores (one in the midfrontal and two in the posterior region), whereas the ventral surface presented two brown chromatophores located one on each side of the funnel. No funnel chromatophores were observed. The dorsolateral surface of the mantle presented —six to seven chromatophores, whereas the ventral surface presented five in the midregion and one toward the posterior end, all of them varying in their expansion (i.e. dots or lines; see Figure 2G).
Family Chtenopterygidae
The only paralarva was identified as Chtenopteryx sp. (Table 1; Figures 2I–K; GenBank accession number: MH347316), agreeing with sequencing results of the mitochondrial gene COI that evidenced a 91% similitude with Chtenopteryx sicula (Vérany, 1851) (GenBank accession number: HQ386019.1). There was no match with any sequence in the BOLD identification system. This individual had a [End Page 168] muscular mantle of 4.5 mm, which was broadly rounded at the posterior end. Head width corresponded to 51% of ML, with eyes around 16% of ML. The funnel was well developed, reaching the midsection of eyes and corresponding to the 23% of ML. The fins were peculiar, consisting of muscular pillars (fin ribs) with the appearance of a comb (i.e. combfin squids; Figs. 2I, J). Arms were shorter than the mantle (31% of ML) and had a uniserial line of —seven to eight pedunculated suckers of similar size. Tentacles were short, with oval clubs of around —six to seven pedunculated suckers (~0.1% of ML) in a circular pad of ~1.5% of ML. The sucker surface was directed toward the front (Figure 2K). Brown/orange chromatophores differentially spotted the dorsal and ventral surfaces of the body, including the aboral surfaces of arms, tentacles, the dorsal and ventral head, and the funnel. The dorsal and ventral mantle lacked pigmentation. A couple of small brown chromatophores were distinguishable in the mid-section of each fin (Figures 2I, J).
Family Enoploteuthidae
Two paralarvae (Table 1) were identified as members of the Enoploteuthid group of families (i.e. Enoploteuthidae, Ancistrocheiridae, Pyroteuthidae, Lycoteuthidae), but their taxonomic status could not be identified to genus or species level.
The smaller individual collected had a tubular mantle of around 2.5 mm in length (Table 1; Fig. 2L, M). The head width corresponded to 26% of ML, whereas the eyes and funnel represented 18% and 14% of ML, respectively. Fins were very small (Figure 2L). Arms corresponded to the 36% of ML, with around 8 pedunculated suckers of similar size (1.2% of ML) arranged in a biserial row. Tentacles were moderately long (60% of ML). The manus showed ~16 pedunculated suckers of around 2% of ML, including other —three to four smaller suckers in the anterior end. No hooks visible (Figure 2M). Chromatophore patterns were distinguished in the aboral and dorsal surfaces of the body. Each arm presented one small brown chromatophore on the tip. Tentacles presented an arrangement of 10 chromatophores each, including five along the stalk and five on the aboral surface of the manus. The dorsal head was almost totally covered by two expanded chromatophores. No supraocular chromatophores were observed. The ventrolateral surface of the head presented two chromatophores located on the left side behind the eye. No photophores or funnel chromatophores were evidenced. The dorsal mantle showed a single brown chromatophore in the central region, whereas the ventral mantle had two chromatophores toward the posterior end (Figure 2L).
The larger paralarvae (2.79 mm ML; Table 1) had a similar morphology compared to the individual described earlier. Head width, eyes, and funnel corresponded to 50%, 18%, and 17% of ML, respectively. Fins were small (Figure 2N). Arms corresponded to 71% of ML (arms IV less developed) and presented 18–19 pedunculated suckers of similar size (2.5% ML) arranged in a biserial row. Tentacles were very long (125% of ML). The manus showed ~16 pedunculated suckers of around 2% of ML and —six or seven smaller suckers on the tip. No hooks were visible (Figure 2O). Brown chromatophores spotted the aboral, dorsal, and ventral surfaces of the body. Arms presented four to six small brown aboral chromatophores, whereas there were 12 in the tentacles arranged as follows: four distributed along the stalk and eight on the aboral surface of the manus. The dorsal head was spotted with — two or three chromatophores. No supraocular chromatophores were apparent. The ventrolateral surface of the head also evidenced two chromatophores on left side next to the eye. No photophores or funnel chromatophores were evident. The dorsal mantle showed around six chromatophores dispersed around the central region and posterior end. The ventral mantle presented twelve chromatophores disposed in the frontal (2), central (2), and posterior (8) region of the mantle (Figure 2N).
discussion
One of the most remarkable features of the water masses around the Rapa Nui Province is its clarity, representing the poorest plankton productivity on Earth (von Dassow and Collado-Fabri 2014). Nonetheless, the [End Page 169] biological importance of this region is supported by the observed increase in coastal phytoplankton biomass when considering the oligotrophic subtropical gyre, which is characterized by very low chlorophyll-a (Chl-a) concentrations and an important dominance of picoplankton and nanoplankton species (Andrade et al. 2014). In terms of field sea surface temperature (SST), there is a gradual increase from the southeast (20.5°C) to the northwest (24°C), with the island being located near the isotherm of 22.5°C. In fact, the region of highest mean temperature coincides with the lowest mean Chl-a values, whereas, in the southeast, the lowest temperature and the highest Chl-a values are observed, indicating an inverse relationship between both variables (Andrade et al. 2014). In this context, the marked dipole in Chl-a concentrations and temperature seems to be reflected in suitable areas for the development of larval stages (Andrade et al. 2014), because most larval stages during these samplings were found on the warmer northwest site.
Studies related to the distribution of larval and postlarval organisms around the Rapa Nui ecoregion have increased during recent years, especially those focused on larval fishes (Castro and Landaeta 2002, Landaeta et al. 2002, 2003, 2005) and crustaceans (Rivera and Mujica 2004, Mujica 2006, Meerhoff et al. 2017). In the specific case of paralarval stages of cephalopods, information is completely lacking, and this study corresponds to the first record for SEP oceanic islands in general, and for the Rapa Nui ecoregion in particular. Currently, most studies have focused on a few continental species and used manipulative approaches in the laboratory [e.g. Robsonella fontaniana (d’Orbigny, 1834), Octopus mimus Gould, 1852, Enteroctopus megalocyathus (Gould, 1852) (Uriarte et al. 2010, 2012, 2016, Zúñiga et al. 2013)] or studied the ecology and distribution of paralarval stages [Dosidicus gigas (d’Orbigny, 1835), Semirossia patagonica (Smith, 1881), R. fontaniana, E. megalocyathus, Doryteuthis (Amerigo) gahi (d’Orbigny, 1835), Onycoteuthidae] (Carrasco et al. 2012, Carrasco and Pérez-Matus 2016, Ibáñez et al. 2015, Pardo-Gandarillas et al. 2016).
Increasing efforts on field surveys around poorly investigated SEP environments, such as Rapa Nui, complement current knowledge on cephalopods’ life-history strategies in different ways. In the case of the octopod fauna recorded, the present morphological and genetic approach provide new information to: (a) support the occurrence of the genus Octopus in coastal areas around the island as previously described by Voss (1979) and (b) clarify that the currently accepted genus, Callistoctopus Taki, 1964 (see Norman and Hochberg 2005), has not been yet identified for Rapa Nui. In fact, the phylogenetic analysis suggested that the juvenile and the paralarvae collected evidenced a 100% similitude between them, and were both closely related with the Indo-West Pacific species Octopus parvus and O. bocki Adam, 1941, enhancing the fact that our individuals belonged to the Octopus group, which is genetically distant from the Callistoctopus group. These results suggest that the specimens could effectively correspond to the endemic species Octopus rapanuiVoss 1979. In terms of morphology, paralarvae collected from Rapa Nui also differed from those described for Callistoctopus sp., including smaller hatching size (1.0 vs. 4.0 mm ML, respectively) and lesser number suckers per arm (three versus seven, respectively) (for details see Sweeney et al. 1992, Villanueva and Norman 2008, Villanueva et al. 2016). Further morphological and genetic studies of different ontogenetic stages are now underway to clarify the life-history traits of this species.
For the family Ommastrephidae, records from SEP waters suggest the marked occurrence of around seven species in the Chilean coast [i.e. Todarodes filippovae Adam, 1975, Martialia hyadesi Rochebrune & Mabille, 1889, Nototodarus hawaiiensis (Berry, 1912), gigas, Sthenoteuthis oualaniensis (Lesson, 1830), Ommastrephes bartramii, and Eucleoteuthis luminosa], with their diversity and distribution being controlled by external physical forcing other than water temperature [End Page 170] (i.e. continental shelf area) that may affect their tolerance limits, especially during planktonic paralarval stages of dispersal (Ibáñez et al. 2009). Specifically, subtropical oceanic waters around Rapa Nui have been identified as potential spawning areas for the latter two species owing to the presence of mature males of O. bartramii (i.e. well developed spermatophores) and recently copulated females of E. luminosa (Prado 1983). The current record of paralarval stages of O. bartramii (i.e. rhynchoteuthions) reinforce this idea, suggesting that these waters might correspond to spawning grounds for this ecologically and economically important species. In fact, considering that tentacles were still fused forming a proboscis, it is plausible to state that spawning had occurred within a period of 2 weeks, as juveniles with separated tentacles are at least 1 month old (Young and Hirota 1990). Around the Hawaiian Archipelago, it has been reported that paralarval stages of this species could disperse over 600 km in 2 weeks (Bower 1996), suggesting that further research should integrate topics related to adult reproductive condition, egg/paralarvae size, and ecology of juvenile forms to better understand dispersal capacities of this and other ommastrephids occurring around Rapa Nui.
In the case of the family Chtenopterygidae, this finding corresponds to the first record from Chilean waters and the Rapa Nui ecoregion. The morphological traits exhibited by this juvenile (i.e. comblike fins, tentacular club) as well as the genetic analyses agreed in identifying this specimen as Chtenopteryx sp.; nonetheless, since genetic analyses evidenced only a 92% similitude with the Mediterranean species Chtenopteryx sicula (the only Genebank sequences available), we suggest that this specimen could correspond to Chtenopteryx sepioloides Rancurel, 1970, a poorly known species that has been described only from the southwestern Pacific (see Jereb and Roper 2010, Sweeney et al. 1992), and for which genetic data is still lacking.
For the Enoploteuthid group of families (i.e. Enoploteuthidae, Ancistrocheiridae, Pyroteuthidae, Lycoteuthidae), records from the SEP coast (i.e. 18° S, 56° S) describe the occurrence of at least six species in Chilean waters [i.e. Lycoteuthis diadema (Chun, 1900), Enoploteuthis semilineata Alexeyev, 1994, Abraliopsis (Pfefferiteuthis) affinis (Pfeffer, 1912), Abraliopsis (Micrabralia) gilchristi Robson, 1924, Ancistrocheirus sp. Gray, 1849, Pyroteuthis margaritifera (Rüppell, 1844)], with only three of them (i.e. L. diadema, A. gilchristi and P. margaritifera) occurring around 27° S (Ibáñez et al. 2009). Despite difficulties in Enoploteuthids systematics, the paralarvae collected in these surveys (< 3 mm ML) evidenced some common morphological traits used to identify early stages of this group of families, including cone-shaped mantles, prominent eyes, well-developed arms and tentacles (except the fourth pair of arms that was very short), arms suckers arranged in a biserial row, and in some species, no apparent photophores in arms, tentacles or eyes (for details see Haimovici et al. 2002, Sweeney et al. 1992, Vecchione et al. 2001, Zaragoza et al. 2015). Considering that only adult stages of these oceanic species have been identified to date from Chilean waters (Ibáñez et al. 2009), further morphological and molecular studies are still needed to clarify the specific identity of the specimens collected in these surveys.
Information on the marine ecosystem around the Rapa Nui ecoregion is rapidly expanding owing to increasing sampling efforts in recent years. Results from previous oceanographic campaigns (e.g. CIMAR Islas Oceánicas 5, 6, 21, 22) and from recent field-based studies (e.g. Easton et al. 2017, 2018, Friedlander et al. 2016, Hernández et al. 2015, Wieters et al. 2014) have substantially increased the information available for this remote oceanic environment. The efforts made in the present survey provided new information on the identification of cephalopods occurring in these waters, while also highlight the necessity of continuing to explore these habitats in order to fill in knowledge gaps on reproductive ecology and dispersive stages (larval, postlarval and juvenile) of most pelagic and benthic species. [End Page 171]
Millennium Nucleus for Ecology and Sustainable Management of Oceanic Islands (ESMOI), Coquimbo, Chile.
Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Coquimbo, Chile.
Current address: Unidad de Ciencias del Mar (UNDECIMAR), Facultad de Ciencias, Universidad de la Repu,blica, Montevideo, Uruguay.
Millennium Nucleus for Ecology and Sustainable Management of Oceanic Islands (ESMOI), Coquimbo, Chile.
Centro de Estudios Avanzados en Zonas Áridas (CEAZA), Coquimbo, Chile.
acknowledgments
Authors would like to thank Dr. Andrea I. Varela (ESMOI, UCN) and MSc. Alina Cifuentes (Universidad Andres Bello) for providing assistance in molecular analyses of paralarvae. Dr. Evie A. Wieters and Dr. Alejandro Pérez-Matus (Pontificia Universidad Católica de Chile) provided samples of juvenile octopuses collected from the field. Special thanks to the captain and crew of the vessel Tokerau (Chilean Navy), as well the fishers for their assistance during sampling. All images were obtained at Sala de Colecciones Biológicas, Universidad Católica del Norte (SCBUCN). Two anonymous reviewers greatly improved preliminary versions of the manuscript.
Footnotes
1. This research was supported by Iniciativa Científica Milenio (NC120030; Millennium Nucleus for Ecology and Sustainable Management of Oceanic Islands, ESMOI). The funding from a FONDECYT postdoctoral grant (#3150419) to E. Meerhoff and a FONDECYT research grant (#11170617) to S.A. Carrasco is also appreciated.