Schimmelmannia (Rhodophyta: Acrosymphytales): First Report of the Genus in Hawai‘i1
The marine red alga Schimmelmannia cf. elegans was identified from samples collected from an aquaculture facility on the island of Hawai‘i based on morphological and molecular data analyses. The genus has never before been reported from the Hawaiian Islands. Awareness of this species is raised given that it has been suspected as introduced in Cape Town, South Africa, and because Hawaiian coastal marine waters are already heavily impacted by introduced algae.
The marine red algal flora of Hawai‘i comprises approximately 370 species (Abbott 1999, Sherwood et al. 2010). Many of these species are diminutive turf algae, and it is rare that a macroalgal taxon is newly reported from the Islands. Conspicuous growths of a large and unfamiliar red seaweed from an aquaculture facility on the island of Hawai‘i were brought to our attention in 2010 for identification, because the plants were suspected to be alien to Hawai‘i. Characterization of the samples using both morphological and molecular analyses confirmed that they represent Schimmelmannia, a genus of red algae never before reported from the Hawaiian Islands.
materials and methods
Approximately two dozen plants of the unknown red alga were collected from aquaculture facilities of the Big Island Abalone Corporation, Kailua-Kona, Hawai‘i (a tenant of the Natural Energy Laboratory of Hawai‘i Authority [NELHA]) by L. Preskitt in February 2010 and again in May 2010 and were shipped by overnight air to the University of Hawai‘i at Mänoa. Plants were growing in outdoor, unshaded tanks with a mixture of deep and surface salt water at approximately 15°C. Several plants were cleaned of epiphytes and frozen at −20°C for DNA extraction, and the remaining plants were pressed as herbarium vouchers (Sherwood Laboratory accession numbers 06948, 08236–08242; several of these vouchers will be deposited in the Bernice P. Bishop Museum in Honolulu). Material associated with voucher 06948 was used for DNA extraction and sequencing. The tank housing the specimens was subsequently destroyed (C. Viljoen, pers. comm.).
Anatomical observations of the plants were made as described in DeClerck et al. (2002) using compound light microscopy. Initial observations allowed taxonomic placement of the samples in the red algal order Acrosymphytales. DNA was extracted and the partial nuclear large subunit rRNA gene (LSU) was polymerase chain reaction (PCR)–amplified using previously published primers and cycling conditions (Conklin et al. 2009). Successful PCR products were purified and sequenced as in Conklin et al. (2009). Sequences were assembled in Geneious 5.4 (Drummond et al. 2011), and 45 additional LSU DNA sequences of representative Rhodophyta were downloaded from GenBank for comparison. Sequences were aligned using MUSCLE in Geneious (Edgar 2004). Maximum parsi mony and maximum likelihood [End Page 529] analyses were implemented using PAUP in the Geneious platform, and a Bayesian estimate of phylogeny was obtained using MrBayes (Huelsenbeck and Ronquist 2001) (details of phylogenetic analyses available from the authors upon request).
results and discussion
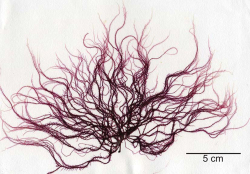
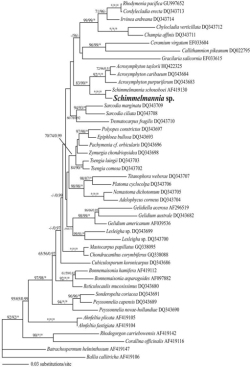
Plants ranged in length from 7 to 20 cm (Figure 1) and were deep wine red in color with a soft but not gelatinous texture. Plants were determined to be uniaxial in construction with a prominent apical cell and a narrow main axis (less than 1 mm wide). Branchlets exhibited bipinnate branching. Plants were not reproductive. Based on overall morphology the plants most closely resembled those of the genus Schimmelmannia, which currently contains eight species. Without details of reproduction, however, species-level identification with microscopy is difficult. Comparisons based on molecular data were undertaken to verify the conclusions based on morphology. Phylogenetic reconstruction based on the LSU marker included all available sequences for the Acrosymphytales and demonstrated a close relationship between the sequence of the unknown red alga and a sequence of Schimmelmannia schousboei (the only species of Schimmelmannia with DNA sequence data available for any marker), with full support (Figure 2), which was consistent with the genus-level identification assigned based on morphology.
Herbarium press of the red alga Schimmelmannia cf. elegans collected from an aquaculture facility on the island of Hawai‘i (Sherwood Laboratory accession 06948). This is the first record of the alga in the Hawaiian Islands.
Yeh and Yeh (2008) tabulated the vegetative and reproductive characters and the known geographical range for each of the eight currently recognized species of Schimmelmannia (S. bollei Montagne, S. dawsonii Acleto, S. elegans Baardseth, S. formosana W.-J. Weh & C. C. Weh, S. frauenfeldii [End Page 530]
Maximum likelihood phylogeny of the red algae based on partial nuclear LSU sequences. Support values for individual nodes are indicated with maximum parsimony bootstrap as the first value, maximum likelihood bootstrap as the second value, and Bayesian posterior probability as the third value. Asterisks indicate full support.
[End Page 531]
Grunow, S. plumosa [Setchell] I. A. Abbott, S. schousboei [J. Agardh] J. Agardh, and S. venezuelensis Ballantine, García, Gomez & M. J. Wynne). Of these, only two have a bipinnate thallus (S. elegans and S. frauenfeldii [DeClerck et al. 2002]), and the overall morphology of the samples most closely resembles that of S. elegans, to which we tentatively assign our specimens (Schimmelmannia cf. elegans). Confirmed species-level identification will require details of reproduction to be observed and/or high DNA sequence similarity with definitive S. elegans material, a species that is currently known only in its native range from the Atlantic island of Tristan da Cunha and Venezuela, and as an introduction to Cape Town, South Africa.
Only one other member of the red algal order Acrosymphytales is known from Hawai‘i: Acrosymphyton taylorii Abbott (Abbott 1999). Members of both Acrosymphyton and Schimmelmannia exhibit a heteromorphic life history with a uniaxial gametophytic thallus and a crustose tetrasporophyte (Yeh and Yeh 2008), which may help to explain how this conspicuous alga arrived in Hawai‘i: it is possible that the species was transported with aquaculture farm stock or feed; however, whether the alga can establish in Hawaiian waters outside the aquaculture facility is not clear. On the one hand, the genus has a probable history of introductions; Schimmelmannia elegans was reported in and around an aquarium tank in Cape Town, South Africa, and it was postulated that the alga was introduced to the area from Tristan da Cunha via a water inlet pipe that was located in close proximity to international fishing vessels (DeClerck et al. 2002). On the other hand, conditions in surface waters around the Hawaiian Islands are not likely to be conducive to the establishment of S. elegans: the species is found in waters ranging from 12°C to 20°C in its native habitat, whereas surface coastal waters in Hawai‘i are typically between 22.8°C and 26.1°C (Zeigler 2002) (water to maintain the abalone aquaculture facility is a mixture of that pumped from approximately 1,000 m depth and thus cold in temperature, and surface waters). In support of this, attempts to grow Schimmelmannia specimens in tanks at the Big Island Abalone Corporation at 12°C–14°C and 18°C–19°C were successful, but plants did not survive at ambient seawater temperatures (24°C–26°C) (C. Viljoen, pers. comm.). In addition, in its native range S. elegans is usually associated with boulders or kelp beds (DeClerck et al. 2002), which are not found in coastal Hawaiian waters.
Because Schimmelmannia likely arrived at the aquaculture facility in a shipment of abalone spat or feed, the heteromorphic life history of the alga may have played a role. Schimmelmannia has a life history that alternates between a crustose tetrasporophyte and a more conspicuous gametophyte (DeClerck et al. 2002). The diminutive tetrasporophyte life history phase was probably the original form of introduction. This scenario proposes that the crustose tetrasporophyte was present on or in the shells of imported abalone stock for some time, with the gametophyte becoming evident (February 2010) with activation of this portion of the life history as day lengths became longer. Establishment of the alga in the aquaculture tank (ca. 15°C) is also consistent with water temperatures of part of the range of the alga: the warm temperate Atlantic island of Tristan da Cunha, and Cape Town, South Africa, where the species became established from the Two Oceans Aquarium. Although grow-out facilities in Hawai‘i for shellfish spat must be periodically monitored for diseases and parasites (V. Nakamoto, pers. comm.), measures are not in place to monitor introductions by other groups of organisms. In addition, abalone aquaculture operations in Hawai‘i do not necessarily need to treat discharge water (V. Nakamoto, pers. comm.).
A number of alien seaweeds have established in Hawai‘i and are causing widespread ecological and economic damage (Smith et al. 2002); thus, awareness of macroalgal species new to the state is critical to allow sufficient monitoring and response. Although it is difficult to predict how a nonnative species will act in a new environment, some other nonnative algal species in Hawai‘i (such as Gracilaria salicornia [C. Agardh] E. Y. Dawson, Acanthophora spicifera [M. Vahl] Børgesen, and Hypnea musciformis [Wulfen] J. V. Lamouroux) [End Page 532] have become established and invasive, resulting in overgrowth of corals and unchecked biomass accumulation (Huisman et al. 2007). This is not the first record of an introduced macroalga from aquaculture operations on the island of Hawai‘i: in April 1987 the red alga Mazzaella volans (C. Ag.) J. Ag. was collected off Keāhole Point near Kona, Hawai‘i, at the outfall of an introduced Macrocystis experimental aquaculture tank (Abbott 1999) (the native range of the species extends from Mexico to Oregon along the Pacific coast of North America). Although the alga was not subsequently re-collected, its presence outside the aquaculture facility indicates that the possibility exists for new seaweed introductions in this area due to aquaculture operations at Keähole Point. Given these reports of nonnative algae in and around Hawaiian aquaculture facilities, we recommend that aquaculture operations in Hawai‘i be required to report the presence of unknown species that grow out in their facilities, and that abalone farms be required to treat discharge water.
Current address: Department of Biology and Environmental Science, University of New Haven, 300 Boston Post Road, West Haven, Connecticut 06516
acknowledgments
We thank Cecilia Viljoen (Big Island Abalone Corporation) and Linda Preskitt (Eyes of the Reef) for bringing this interesting collection to our attention. We thank Katherine Cullison (Hawai‘i Division of Aquatic Resources) and Vernon Nakamoto (Hawai‘i Department of Agriculture) for helpful discussions. Celia Smith (University of Hawai‘i) provided helpful comments on an early draft of the manuscript.
Literature Cited
Footnotes
1. Manuscript accepted 26 January 2012.