Nectar Production by Invasive Lantana camara and Endemic L. peduncularis in the Galápagos Islands1
Measurements of nectar volume and sugar concentration along two elevational gradients, in flower exclusion experiments, and over a diurnal cycle of nectar production were compared for invasive Lantana camara and endemic Lantana peduncularis in the Galápagos Islands during the dry season. Both species show the same pollination syndrome and are pollinated by Lepidoptera. Lantana camara flowers had a higher nectar volume than L. peduncularis flowers at every elevation and higher total sugar content. However, nectar in L. peduncularis flowers was much more concentrated than in L. camara flowers at lower elevations. The differences in nectar production between Lantana species seemed to be intrinsic and related to contrasting strategies to cope with drought. Nectar volume of unbagged flowers of L. camara was lower than that of bagged flowers at sunrise, which was probably related to consumption of nectar by nocturnal Lepidoptera. Nectar removal by floral visitors had a pronounced effect on total amount of nectar secreted by L. camara flowers but sugar concentration did not vary significantly. Our results suggest that L. camara may compete with L. peduncularis for pollinators.
Flowering plants have developed several strategies to attract pollinators and ensure repeated visits that lead to pollination (Simpson and Neff 1983), with pollen and nectar being the most common rewards offered by plants to their pollen vectors (Faegri and van der Pijl 1979, Proctor et al. 1996). The behavior of pollinators (visit frequency and movement between flowers) is influenced by the quality and quantity of rewards offered by flowers (Waser 1983, Real and Rathcke 1988).
The threat to global biodiversity from the spread of invasive plants is all too apparent, and this spread appears to be facilitated by disturbances and by changes in foraging behavior of insect pollinators following the invasion (Ghazoul 2002). Competition for pollination may be particularly strong between native and invasive plants, especially congeners that share flower traits, because there has been less opportunity for selection of divergence in traits, such as flowering time, which may reduce competition (Montgomery 2007). Sympatric plants that share pollinators may compete for those pollinators, often leading to the evolution of temporally staggered nectar production among plant species that minimizes competition for pollinator visits (Willmer and Corbet 1981). Invasive plants with showy flowers and more nectar and pollen production can exert indirect pressure on native plant species by competing for pollinators, significantly reducing seed set of native congeners (Brown et al. 2002) and even other sympatric native species (Grabas and Laverty 1999, Chittka and Schürkens 2001; Morales and Traveset 2009). Competition for [End Page 435] pollinators between invasive and native plant species can take effect even before competition for other resources (Chittka and Schürkens 2001).
Plants growing in arid ecosystems are strongly limited by water availability, affecting both plant growth and reproduction (McKenna and Houle 2000). Nectar production has been identified as a costly floral attribute that may consume a substantial fraction of a plant’s photosynthetic assimilates (Pyke 1991), representing an important investment for plants (Jersáková and Johnson 2006), which may decrease their growth and survival (Golubov et al. 2004).
Lantana camara L. (Verbenaceae), a shrub from tropical America, is considered one of the 10 most noxious weeds in the world (Sharma et al. 1988). It is invading many islands in the Pacific, such as Hawai‘i and the Galápagos. In the Galápagos, L. camara has invaded thousands of hectares from the semi-arid lowlands to higher and more humid elevations (Cruz et al. 1986). In some areas, L. camara grows together with the Galápagos endemic Lantana peduncularis Andersson (Hamann 2004), showing very contrasting strategies to cope with drought (Castillo et al. 2007). This situation offers an unusual opportunity to analyze nectar production of these closely related species along an elevational gradient of water availability (Itow 1992).
The aim of this work was to study the nectar production and its sugar concentration in L. camara and L. peduncularis along an elevational gradient and over the diurnal cycle in the Galápagos dry season. Although previous studies have documented differences in nectar traits and volume among species of the same family or genus (Guitián et al. 1993, Ortega and Devesa 1993, Petanidou and Vokou 1993), none compares nectar traits between native and invasive congeners that share floral characteristics, which might increase competition for pollinators (Brown et al. 2002). Moreover, studying nectar production along an elevational gradient allows us to evaluate how these related species respond to differential water availability.
materials and methods
Study Sites
The Galápagos Islands are located in the Pacific Ocean approximately 1,000 km west of Ecuador. Their vegetation is strongly zoned by elevation because most rainfall is orographic (Alpert 1963, Wiggins and Porter 1971). The Dry Zone, where the most of the archipelago’s endemic plant species grow, is characterized by endemic tree cacti of the genera Opuntia and Jasminocereus and a shrub layer or by open woodland. The Transition Zone is characterized by closed mixed forest, and the Humid Zone includes forests of Scalesia pedunculata and Zanthoxylum fagara and open vegetation dominated by ferns, sedges, and grasses (McMullen 1999). Lantana species are found from the dry lowlands to the Transition Zone (Castillo et al. 2007).
Two distinct seasons can be distinguished during the year in the Galápagos (Trueman and d’Ozouville 2010). The warm season (January to June) is caused by warm ocean currents sweeping southward from Central America. Mean daily maximum temperature is 29°C, and mean daily temperature is between 25°C and 26°C (Ziegler 1995). During this season, the skies are usually clear but heavy showers are frequent; this season is thus the wetter season in the Dry Zone of the Islands. The cool dry season (July to December) is caused by the Humboldt Current, resulting in cooler air temperatures (18°C–26°C), with skies usually overcast. A mist layer, known locally as “garúa,” frequently occurs at higher elevations, but little precipitation occurs in lowlands (Ziegler 1995); this season is therefore the dry season in the lowlands (Trueman and d’Ozouville 2010).
Our study analyzed variation along the elevational gradient of the Galápagos, sampling along two altitudinal transects in the south of Santa Cruz Island: transect 1 from 28 to 253 m above sea level (a.s.l.) along the road to El Garrapatero (0° 39′ 59.72″–0° 41′ 18.51″ S, 90° 15′ 44.92″–90° 13′ 22.95″ W) and transect 2 from 21 to 149 m above sea level, along [End Page 436] the road from Puerto Ayora to Bellavista (0° 42′ 10.02″–0° 44′ 29.62″ S, 90° 19′ 35.50″–90° 19′ 27.59″ W). In addition, a sampling point in the Transition Zone at 75 m a.s.l. (0° 43′ 30.51″ S, 90° 19′ 38.86″ W) was selected to study L. camara nectar production during the diurnal cycle.
Study Species
Lantana camara is a shrub to 3 m tall that invades a wide variety of habitats in tropical, subtropical, and temperate regions (Holm et al. 1977, Sharma et al. 1988). Lantana camara was introduced to the Galápagos as an ornamental species in 1938 (Tye 2001). It covered more than 2,000 ha in 1987 (Lawesson and Ortiz 1990), and it is one of the most important invasive plant species in the Galápagos archipelago. It produces a large number of tubular flowers grouped in flower heads that open sequentially from the outside toward the center (Swarbrick et al. 1998). Newly opened flowers usually have yellow throats, with the flower heads colored in combinations of yellow, orange, red, purple, or pink. These colors tend to change with flower age (Conn 1992, Swarbrick et al. 1998, Parsons and Cuthbertson 2001). Initial studies of Barrows (1976) concluded that L. camara flowers did not self-pollinate, and Mohan Ram and Mathur (1984a) determined that L. camara is self-compatible but needs insects for pollination. However, Neal (1999) found that individual flowers were capable of self-pollination. This invasive species is mainly pollinated by Lepidoptera (Schemske 1976, Hilje 1985).
Lantana peduncularis is an endemic shrub up to 2 m tall that grows mostly in the dry lowlands, although it can also be found in the Transition Zone of the Galápagos Islands. It often forms dense thickets, which are difficult to penetrate, especially during the cool dry season when the plants are stiff and dry. Flowers are tubular and white, often with a yellow throat (McMullen 1999), and are grouped in axillary heads. Lantana peduncularis in flower exclusion experiments was not able to self-pollinate (J.C.-T., unpubl. data).
Abiotic Environment
Soil water content (%) was estimated at each sampling point from a linear regression model relating soil water content to elevation that was constructed by collecting a sample of 100 g of soil between 0 and 5 cm deep at different elevations along both elevational transects (n = 14 locations coinciding with nectar sampling points). Each soil sample was weighed fresh and reweighed after drying it for 72 hr at 80°C. Air temperature (°C) and relative humidity (%) were measured at 1.5 m above the soil surface with a portable thermohygrometer (Elka FTM-10) at every sampling elevation.
Nectar Measurements
Sugar concentration (%) was measured with a pocket refractometer (Brix scale 0%–32% [Zuzi ECO series]), and nectar volume was measured by determining the length of the liquid column within microcapillary tubes of 1 µl (Dafni et al. 2005). When the nectar volume was lower than 0.5 µl, the refractometer was not able to measure sugar concentration; in such cases, 1 µl of distilled water was added to the nectar before measuring the sugar concentration, and results were then corrected for dilution.
Measurements were carried out around midday (1000–1400 hours) on young flowers located at the center of the inflorescence of plants of both species chosen at random (18 plants for L. peduncularis and 16 plants for L. camara) during the dry season in August 2009, along both transects. At each elevation four samples per plant of L. camara and one to two samples per plant of L. peduncularis were measured. Preliminary observations showed that both species (especially L. peduncularis) produced very low nectar volume, and that nectar volume and sugar concentration were almost constant for different flowers of the same plant. For this reason, each sample of nectar (ca. 0.5–1.0 µl) contained the nectar of one to three flowers for L. camara and up to 20 flowers for L. peduncularis. Once the nectar volume and its sugar concentration were [End Page 437] measured, mean values per flower were obtained by dividing each sample by the number of flowers used for that sample.
At intermediate elevations of transect 1 (between 160 and 200 m a.s.l.), eight inflorescences distributed among four plants (chosen at random) of each Lantana species were bagged with a fine nylon mesh, and nectar production and sugar concentration were measured after 24 hr. In addition, nectar production was compared between young yellow flowers located at the center of the inflorescence and old pink flowers at the periphery.
For L. camara, nectar production was measured in unbagged flowers at three different times of the day (0600 hours, 1200 hours, and 1800 hours; n = 20 samples from randomly distributed flowers in five plants chosen at random). In addition, inflorescences from the same plants were bagged at sunset of the previous day, and nectar production was then measured during the diurnal cycle (n = 20 samples from randomly distributed flowers in the same five plants).
To estimate energy values of nectar to consumers, the sugar concentration (%) was converted to mass of sugar per unit volume (mg µl–1) by the formula:
Y = 0.00226 + (0.00937X) + (0.0000585X 2),
where Y represents the mass per unit volume (mg µl–1) and X the percentage of sugar concentration determined by the refractometer. To obtain the total amount of sugar (milli-grams) present in the nectar, the resulting values were multiplied by the nectar volume, according to Galetto and Bernardello (2005).
Statistical Analyses
Statistical analyses were carried out using SPSS v.18 (Statistic Inc.). Data were tested for normality with the Kolmogorov-Smirnov test and for homogeneity of variance with the Levene test. Student’s t-test was used to compare nectar traits between bagged and unbagged inflorescences, and between young and old flowers. One-way repeated measures analysis of variance (ANOVA; F-test) was used to compare nectar volume, sugar concentration, and sugar content during the day in bagged and unbagged inflorescences. If the F-test was significant at the 0.05 probability level, Bonferroni pairwise comparisons were performed to detect differences between times of the day. The Pearson coefficient was used to assess correlations between nectar volume and sugar concentration along the elevational gradient and during the day, and between nectar volume and sugar concentration and elevation. Deviations were calculated as the standard error of the mean (SEM).
results
Nectar Production along the Elevational Gradient
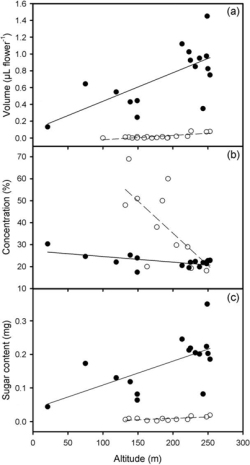
During our work, L. camara bloomed throughout the whole gradient, but L. peduncularis did not flower at elevations below 100 m a.s.l. (Figure 1). In general, L. peduncularis had nectar only in newly opened flowers (with a yellow throat and located at the inflorescence center), and many such flowers had no nectar at all. In fact, all flowers examined at 100 m a.s.l. had no nectar. At higher elevations flowers without nectar were less frequent but still common (up to 46% at 253 m a.s.l.). In contrast, most yellow flowers of L. camara at the inflorescence center had nectar, and ca. 65% of old pink flowers at the periphery of inflorescences had no nectar. Peripheral pink flowers with nectar had a lower sugar concentration (ca. 13%) compared with yellow flowers (ca. 25%) (t = −2.221, df = 8, P < .05).
Nectar volume per young central flower increased with elevation in both species (L. peduncularis: r = 0.77, P < .001, n = 18; L. camara: r = 0.67, P < .01, n = 16), varying between 0.0 and 0.1 µl for L. peduncularis and between 0.1 and 1.5 µl for L. camara (Figure 1a). At the same time, sugar concentration for L. peduncularis decreased as nectar volume increased (r = −0.68, P < .05, n = 12) but not for L. camara (r = −0.48, P > .05, n = 16). Sugar concentration varied along the elevational gradient between 18% and 69% for L. peduncularis and between 18% and 30% for L. camara, decreasing at higher elevations (r = −0.70, P < .05, n = 12; r = −0.62, P < .05, [End Page 438]
Variation of nectar volume (a), sugar concentration (b), and total sugar content (c) for L. camara (0) and for L. peduncularis (4) along the elevational gradient on Santa Cruz Island during the cool dry season, 2009.
[End Page 439]
n = 16, respectively) (Figure 1b). Total sugar content for L. peduncularis varied between 0.002 and 0.019 mg flower–1 and between 0.04 and 0.35 mg flower–1 for L. camara, both increasing with elevation (r = 0.61, P < .05, n = 12; r = 0.63, P < .01, n = 16, respectively) (Figure 1c). Total sugar content increased with nectar volume for both species (P < .0001).
Soil water content varied between 1.3% and 33.3%, increasing with elevation (r = 0.85, P < .001, n = 14). For both Lantana species nectar volume increased (L. peduncularis: r = 0.76, P < .001; L. camara: r = 0.67, P < .01) and sugar concentration decreased with soil water content (L. peduncularis: r = −0.70, P <.05; L. camara: r = −0.60, P < .05). Air temperature oscillated between 25°C and 30°C and the air relative humidity between 53% and 80%, being independent of elevation. Nectar traits were independent of atmospheric conditions (air temperature and relative humidity) for both Lantana species.
At intermediate elevations (160–200 m a.s.l.), no significant differences were found in the sugar concentration of bagged and unbagged inflorescences of L. peduncularis (ca. 23%) (t-test, P > .05) nor in L. camara (ca. 24%) (t-test, P > .05). Nectar volume in bagged and unbagged inflorescences of L. peduncularis was similar (t-test, P > .05). In contrast, in L. camara, nectar volume in bagged inflorescences (0.4 ± 0.1 µl [mean ± SEM]) was higher than in unbagged inflorescences (0.3 ± 0.0 µl; t = −2.501, df = 6, P < .05).
Diurnal Cycle of Nectar Production
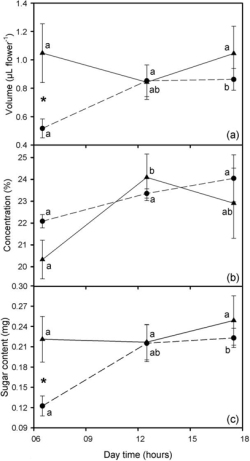
The nectar volume of bagged flowers of L. camara did not change significantly during the day, oscillating between 0.9 and 1.1 µl (repeated measures ANOVA F-test, P > .05). In contrast, the nectar volume of unbagged flowers increased during the day (repeated measures ANOVA, F = 4.862; df = 2, 8; P <.05) (Figure 2a). At sunrise, the nectar volume of unbagged flowers was lower than that of bagged flowers (0.5 ± 0.1 µl flower–1 and 1.0 ± 0.2 µl flower–1, respectively; t = 2.439, df = 8, P < .05) (Figure 2a).
Sugar concentration of bagged flowers varied between 20% and 24%, being slightly higher at midday than at sunrise (repeated measures ANOVA, F = 5.157; df = 2, 8; P <.05; paired t-test, P < .05). Sugar concentration of unbagged flowers did not change significantly during the day (repeated measures ANOVA, P > .05) (Figure 2b). No significant differences were found between sugar concentration of bagged and unbagged flowers at each of the three times of the day (t-tests, P > .05) (Figure 2b).
Total sugar content of bagged flowers did not vary significantly during the day (repeated measures ANOVA, P > .05). In contrast, the total sugar content of unbagged flowers was lower at sunrise (0.12 ± 0.01 mg) than at sunset (0.22 ± 0.01 mg) (repeated measures ANOVA, F = 5.910; df = 2, 8; P < .05; Bonferroni’s test, P < .01). Total sugar content was lower for unbagged than for bagged flowers at sunrise (t = 2.675, df = 8, P < .05) (Figure 2c).
discussion
This study shows that invasive L. camara has a higher nectar production than endemic L. peduncularis in the Galápagos Islands. In both species, we found differential nectar production related to the age of the flower. Young yellow flowers of L. camara always contained nectar, but most of the pink flowers were empty. In this species, flower color changes with age, turning from yellow to pink or red (McMullen 1999), a change that is perhaps stimulated by pollination (Mohan Ram and Mathur 1984b). Thus, these differences in flower color could direct pollinators to unpollinated flowers (Weiss 1995). Flowers of L. peduncularis do not show flower color changes as dramatic as those of L. camara, but we observed that only young flowers with a yellow throat had nectar. Thus, the yellow color in the throat could also act as a cue to direct pollinators to the flowers with nectar.
The nectar production of both Lantana species and even the flowering of L. peduncularis seemed to be limited by water stress along the elevational gradient in water availability, which is probably the most important [End Page 440]
Variation during the day in nectar volume (a), sugar concentration (b), and total sugar content (c) in bagged (▲) and unbagged flowers (●) of L. camara at 75 m a.s.l. during the cool dry season, 2009, Santa Cruz Island. * denotes significant differences of nectar volume between bagged and unbagged flowers (t-test), and different letters (a, b) indicate differences during the day (ANOVA for repeated measures and Bonferroni’s test when necessary). Data are mean ± SEM (n = 5).
[End Page 441]
stress factor in the semiarid Galápagos lowlands (Hamann 2004). The limitations were much higher in L. peduncularis than in L. camara, which may be related to the strategy of the former species to cope with drought. Lantana camara has deep roots and behaves as a drought-avoiding species whereas L. peduncularis has a shallow root system, behaving as a drought-tolerant species, suffering high water stress during the dry season (Castillo et al. 2007). Thus, L. peduncularis was not even blooming at the lowest elevations. At middle elevations every sampled flower had no nectar. In contrast, L. camara bloomed and accumulated nectar at every elevation, although its nectar production at the lowest elevation was less than 10% of that at the highest (from 0.1 to 1.5 µl).
Although the nectar sugar concentration of L. camara was lower than that of L. peduncularis, its nectar production was higher. Therefore, L. camara produced more sugar content per flower at every elevation. The low nectar accumulation recorded for the endemic L. peduncularis was not due to its consumption or evaporation but to a low nectar production rate, as shown by the exclusion experiment. The low nectar production with high sugar concentration recorded for L. peduncularis may be an adaptation to poor pollinator conditions in oceanic islands (Elmqvist et al. 1992) as well as to small-bodied pollinators that are content to visit flowers with low nectar volume if they do not have to compete with large-bodied pollinators (Schaffer and Schaffer 1979, Collevatti et al. 1997).
Nectar removal by floral visitors had a pronounced effect on the total amount of nectar secreted by L. camara but not on its sugar concentration, in contrast to some other species (Pyke 1991, Galetto and Bernardello 1992, 2004, Guitián et al. 1995, Castellanos et al. 2002). The sugar concentration for both Lantana species increased at lower elevations, being, in general, more concentrated in L. peduncularis. Higher sugar concentration at lower elevations could be related to the production of more-concentrated nectar but less nectar volume as a response to drought stress (Carroll et al. 2001) and /or to a higher nectar evaporation rate due to higher flower temperature (Nepi et al. 2001, Corbet 2003). Nectar volume and total sugar content for both Lantana species increased according to the soil water content in the Galápagos. This result is similar to the findings of Wyatt et al. (1992), who showed that nectar volume and sucrose amounts of Asclepias syriaca were increased after watering, and those of Petanidou et al. (1996), who showed that nectar secretion of Capparis spinosa L. depended on precipitation.
Both Lantana species have long, narrow corolla tubes with nectar, which are traits commonly associated with butterfly pollination (Faegri and van der Pijl 1979, Lewis and Lipani 1990, Weiss 1995, Proctor et al. 1996). In fact, we have observed that both Lantana species share diurnal Lepidoptera as pollinators (J.C.-T., pers. obs.). The diurnal cycle of nectar production of L. camara showed that nectar volume in unbagged flowers was lower than in bagged flowers at sunrise, and that nectar volume of unbagged flowers increased progressively during the day, reaching a similar nectar volume at midday as that of bagged flowers. These results suggest that the highest nectar consumption by pollinators of L. camara occurred during the night. In fact, moths visiting L. camara flowers were observed (J.C.-T., pers. obs.).
Our study shows that L. camara, one of the most invasive plant species on earth, produces much more nectar and more sugar than the Galápagos endemic L. peduncularis. Taking into account that floral nectar is probably the key reward offered by Lantana to their pollen vectors, and that the behavior of pollinators can be influenced by the quality and quantity of such rewards (Waser 1983, Real and Rathcke 1988), the alien species with its higher energetic quality may represent a strong competitor with the endemic for pollinators, especially for large-bodied pollinators with higher energetic demands (Watt et al. 1974). To collect the same total amount of sugar present in a single flower of L. camara, pollinators would have to visit ca. 20 flowers of L. peduncularis, which would represent a greater energetic expense. Taking into account that both Lantana species share the same pollination syndrome including flower traits such as [End Page 442] shape, size, and reward type (nectar), and that both species are pollinated by Lepidoptera (J.C.-T., pers. obs.), the differences in nectar production between the species may result in pollinator competition, as occurs in other congeneric and sympatric species (Berjano et al. 2009), which may affect the reproductive success of L. peduncularis.
acknowledgments
Thanks to the staff of the Galápagos National Park for their assistance and to Mabel González and Tania Quisingo for their help in the field.
Literature Cited
Footnotes
1. This research was supported by “Agencia Española de Cooperación Internacional para el Desarrollo” (AECID) through a grant to J.C.-T. and by “Plan Propio de Investigación” of the University of Seville. Manuscript accepted 7 December 2011.